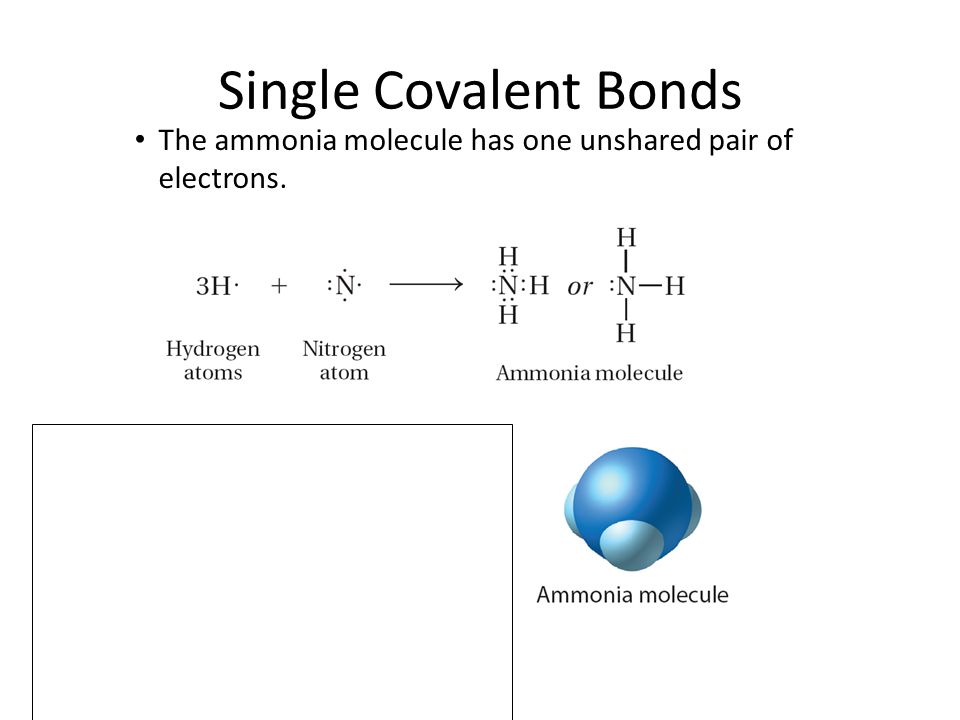
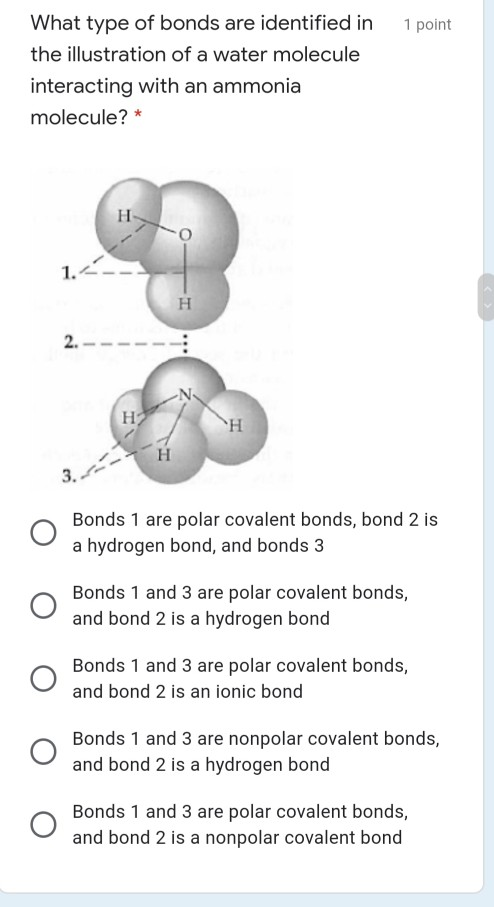
38 the ammonia molecule in the diagram has the observed bond orientation because ...
A molecule of ammonia, with its pyramidal structure and each N-H bond being 1.01 Å long (the size can be taken to be 1.01 Å), would fit in easily within the confines of \(\hbox {C}_{60}\), which has a radius of 3.53 Å and inside \(\hbox {C}_{50}\), which has a radius of 3.23 Å. The radius of the cage here is taken to be half the distance...
PF5 Lewis structure, Molecular Geometry, Bond angle and Shape. Phosphorus Pentafluordie is a colourless and toxic gas. It is made up of one Phosphorus atom and five Fluorine atoms. This molecule is also known as the halide gas as it consists of Fluorine (a halogen atom). To understand the physical and chemical properties of this molecule, it is...
The researchers emphasized, "This study is the first case of explaining the observation of the dynamics of water molecules in superconcentrated aqueous electrolytes at a molecular level," and "It is possible because IR-PP has the ability to distinguish and observe water molecules according to their hydrogen-bonding partner."

The ammonia molecule in the diagram has the observed bond orientation because ...
Feb 8, 2021 — The ICl3 molecule will have a T-shaped molecular geometry...The Lewis structure of ICl3 is a drawing or model chemists use to predict the .... The geometry of ICl3 is trigonal bipyramidal with a T-shaped molecular shape. ICl3 has three bond pairs and two lone pairs of electrons.
H-bond cooperativity is important, because it often entails mutual strengthening of the H-bonds, and because it can confer specific properties to the molecule [71,72,73,74,75,76]. It is not easy to evaluate the energy of IHBs, because the removal of an IHB causes major changes in the molecule's geometry and, therefore, the energy change...
Interestingly, hydrogen-bonding interactions between equatorial chp ligands and an incoming ammonia molecule (N ⋯ O distances of 2.61 and 2.62 Å) are calculated to play a role in facilitating...
The ammonia molecule in the diagram has the observed bond orientation because ....
E-H bonds are simply a way of denoting any bonds you might make between hydrogen and another element. E-H bond strengths are highly dependent on the chemical structure of each element, but many of these bonds are weak — unstable and inclined to break easily and form hydrogen (H 2). Most chemical reactions are driven by the formation of strong...
A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom. In the formation of a simple or ordinary covalent bond, each atom supplies at least one electron to the formation of the bond - but that is not the case every time.
Ammonia is a compound of nitrogen and hydrogen with the formula NH 3.A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. It is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45 percent of the world...
NO is another nitrogen-containing gas molecule, as a free radical which has an unpaired electron in the 2p* antibonding orbital [16,17], thus the N O bond is easy to activate. In recent years, some scholars have tried to use NO as a nitrogen source to synthesize ammonia through electrocatalysis which is named electrocatalytic nitric oxide...
Ammonia has a molecular dipole moment of 14.7 D which denotes the degree of separation between the positively and negatively charged particles. In contrast, HCl has a dipole moment is 1.08 and the water molecule has a dipole moment of about 1.85. Due to this charged polarity ammonia has limited and minimal permeability through lipid membranes.
Furthermore, the shape of the frontier molecular orbitals of Au \(_{79}\) and of the incoming organic molecules has been found to dictate the preferred orientation of the adsorption. Several quantum chemical topological analyses of the electron density have been performed to further classify the interactions to weak dispersive or van der Waals...
Answer (1 of 2): https://en.wikipedia.org/wiki/Ammonium Usually, the ion has 8 electrons in the valence shells, 5 from nitrogen and 3 from the four hydrogens. Example...
Various studies [39,40] agree on a hydrogen-bonded structure with the ammonia molecule donating its lone pair of electrons and the water molecule accepting them with one of its OH groups. The hydrogen bond is almost linear, but the ammonia molecule is significantly tilted and the complex is dynamic.
The possibility of one water molecule as a catalyst in reactions of OH + CH 3 OH, have been proposed by Jara et al. 12,13 and Chao et al. Chao et al. 11 have measured reaction rate coefficients...
The molecule has a six-coordinate structure,...direction of the hydrogen bonds 29 because the water molecule acts as a donor and...The molecular orientation is observed to slightly vary after...
The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. The Lewis structure of sulfur trioxide (SO3) molecule is drawn by: First, look for the total number of valence electrons in a single sulfur trioxide (SO3) molecule, which is twenty-four.
The reaction of ammonia with an acid to produce ammonium salt fits in the examples of a coordinate covalent bond formation. The nitrogen atom of ammonia has an electron pair (Lewis base) that isn't involved in bonding as long as its ammonia.
The shorter bond length has greater bond strength. Based on the bond length, covalent bonds are of the following types. Single Covalent Bonds Between Different Atoms. The simplest covalent bond exists in the diatomic hydrogen molecule. Halogens also exist as diatomic gases by forming covalent bonds, such as chlorine.
Key Terms. substrate: A reactant in a chemical reaction is called a substrate when acted upon by an enzyme.; induced fit: Proposes that the initial interaction between enzyme and substrate is relatively weak, but that these weak interactions rapidly induce conformational changes in the enzyme that strengthen binding.; active site: The active site is the part of an enzyme to which substrates...
The N—H bond in ammonia is quite polar on account of the electronegativity difference of N (3·0) and H (2·1). On the contrary, P—H bond in phosphine is almost non-polar because both P and H atoms have almost same electronegativity (21). Due to polarity, intermolecular hydrogen bonding is present in the molecules of ammonia but not in...
by AV Burenin · 2012 · Cited by 27 — intramolecular dynamics since the first part of the book is dedicated to it. For a more ... of the NH3 molecule has the symmetry group C3v at once.
The ammonia trimer has a cyclic structure (labeled 3-I) with slightly shorter hydrogen bonds than the ammonia dimer. These results are in accord with previous studies. 12 , 13 , 15 , 16 , 19 The CCSD/AVDZ calculated harmonic vibrational spectra of the ammonia dimer and trimer are shown in Supporting Information Figures S6 and S7 , respectively.
Most of hydrogen-bonded molecules exhibit to a larger or lesser extent all of ... if the equilibrium geometry has a nearly linear X-H⋯Y shape, and the bond ...
This is because a multiple bond has a higher electron density than a single bond, so its electrons occupy more space than those of a single bond. For example, in a molecule such as CH 2 O (AX 3 ), whose structure is shown below, the double bond repels the single bonds more strongly than the single bonds repel each other.
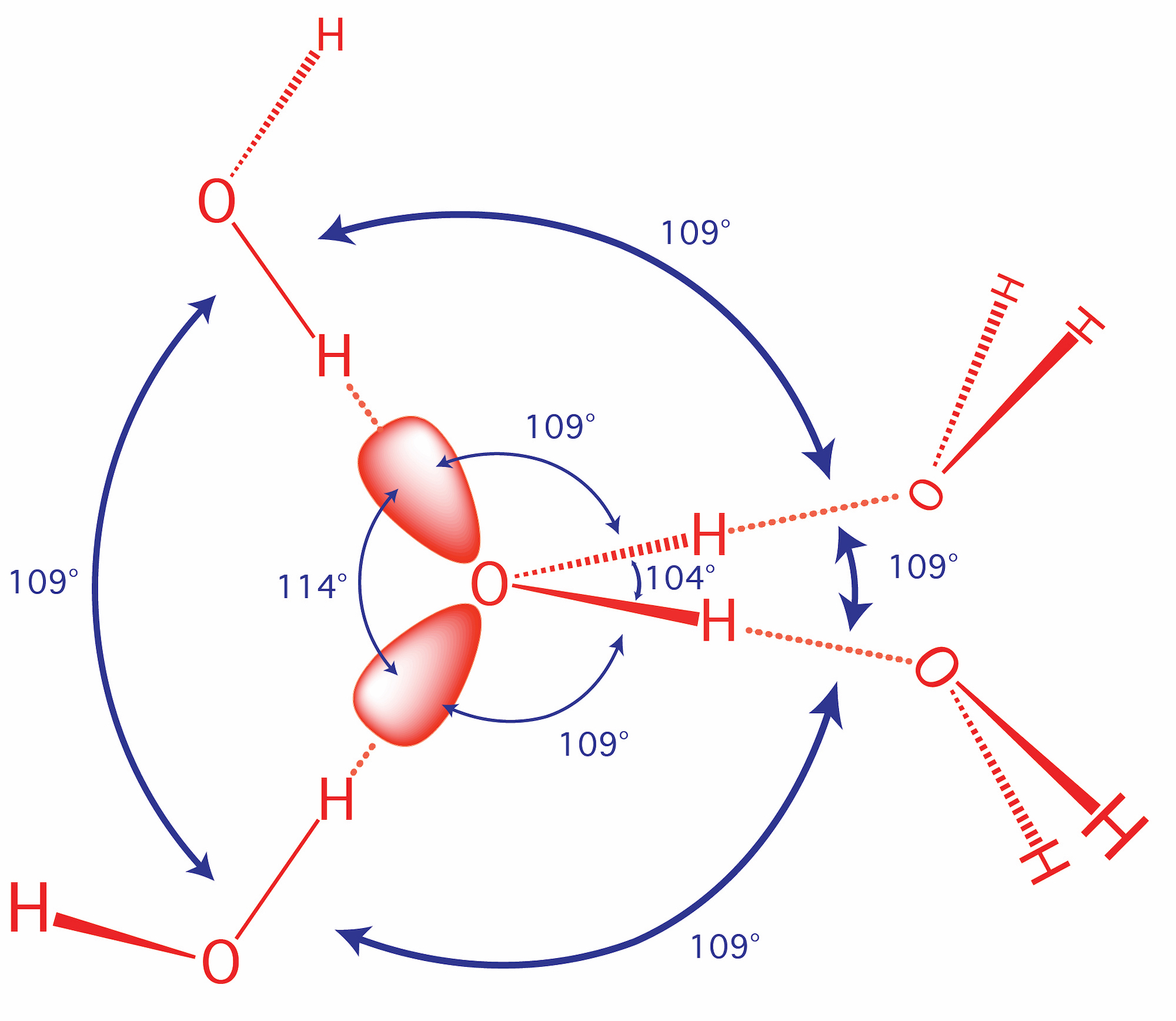
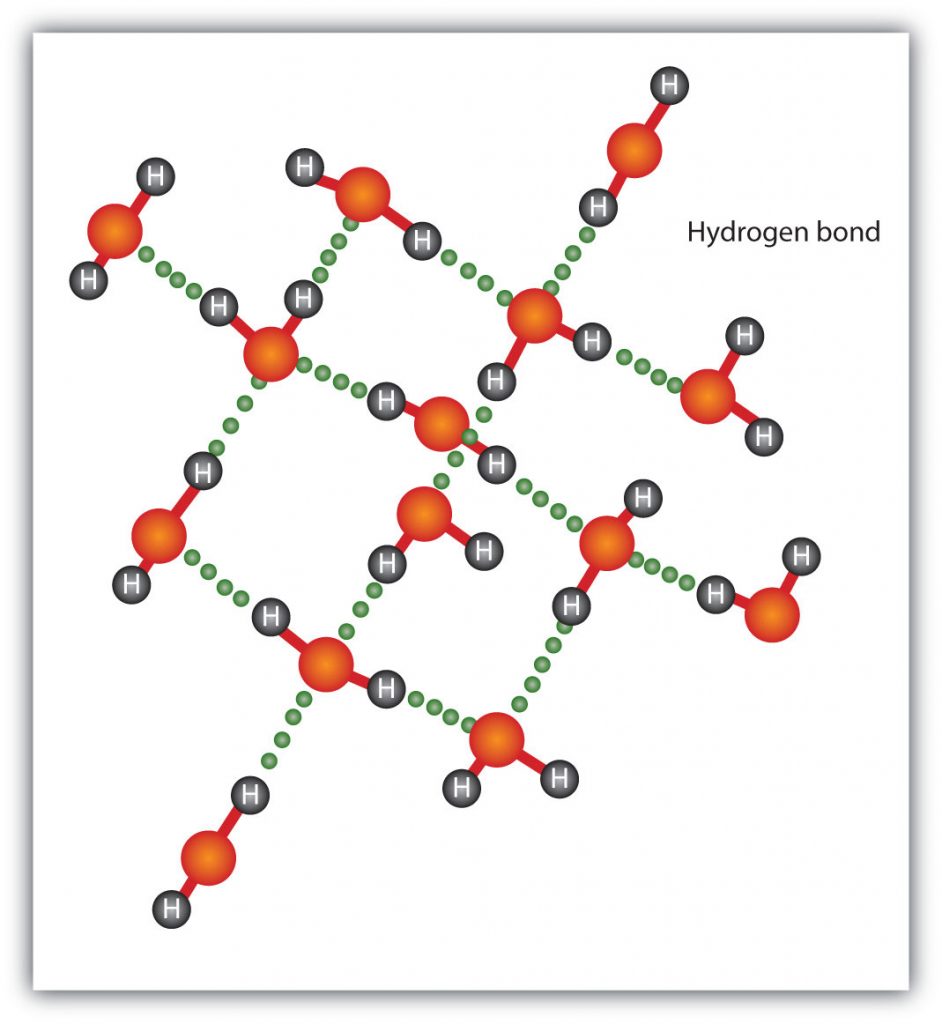
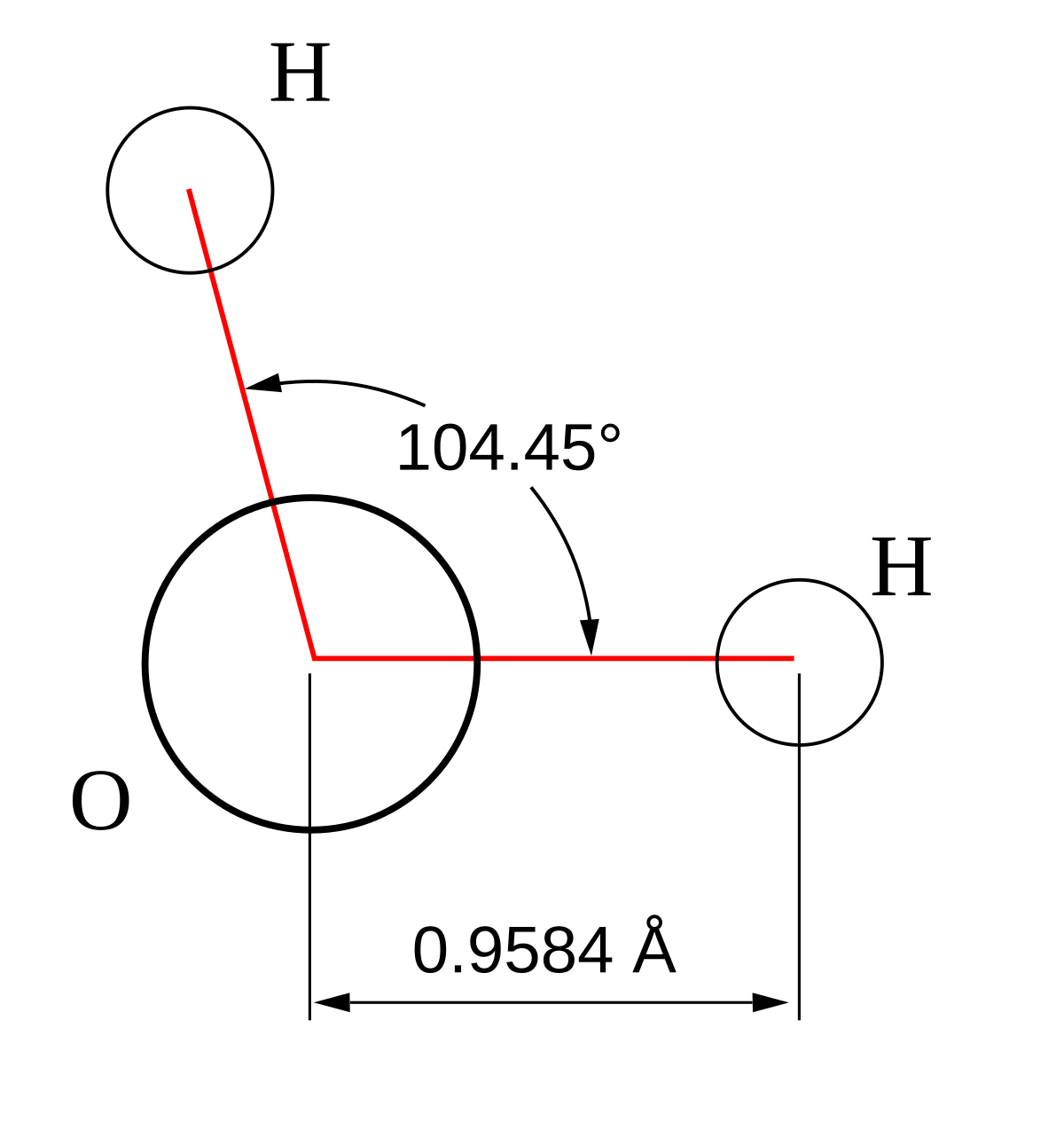
The attraction between individual water molecules creates a bond known as a hydrogen bond. See Fig. 3-7. A molecule of water has two hydrogen atoms. Both of ...
This is because a multiple bond has a higher electron density than a single bond, so its electrons occupy more space than those of a single bond. For example, in a molecule such as CH 2 O (AX 3 ), whose structure is shown below, the double bond repels the single bonds more strongly than the single bonds repel each other.
by H Zabel · 1988 · Cited by 6 — are surrounded on the average by four NH3 molecules, and the remaining molecules are essentially free. Quasi-elastic neutron.
A neutral nitrogen atom has five valence electrons (it is in group 15). From its Lewis electron structure, the nitrogen atom in ammonia has one lone pair and shares three bonding pairs with hydrogen atoms, so nitrogen itself is assigned a total of five electrons [2 nonbonding e − + (6 bonding e − /2)]. Substituting into Equation \(\ref{8.5...
by HSP Müller · 2010 · Cited by 17 — This has prompted the present reinvestigation of the H2DO+ spectra , which ... even triply deuterated ammonia, ND3, and methanol, CD3OH, have been observed, ...
The Ammonia Molecule In The Diagram Has The Observed Bond Orientation Because The electrons form 3 bonds and 1 lone pair of electrons. N has four pairs of electrons in the valence shell b.
by MJ Schultz · 2002 · Cited by 269 — ammonia-water complex is observed with ammonia tilted, on average, 25-38° from the normal. ... aqueous glycerol surface because both molecules are indepen-.
In the ammonia molecule, for example, the nitrogen atom normally has three unpaired p electrons, but by mixing the 2 s and 2 p orbitals, we can create four sp3 -hybrid orbitals just as in carbon. Three of these can form shared-electron bonds with hydrogen, resulting in ammonia, NH 3. The fourth of the sp3 hybrid orbitals contains the two...
by Y Filinchuk · 2009 · Cited by 66 — and B atoms and the orientation of NH3 and BH3 groups were finally assigned ... N bond changes the eclipsed conformation of the NH3BH3 molecule into the ...
The diagram showing orbital overlapping in the ammonia (NH3) molecule. The orbitals of NH3 participating in the bond formation to undergo sp3 hybridization . Molecular orbital diagram of ammonia (NH3) molecule. The molecular orbital diagram is a diagrammatic representation of how chemical bonding is taking place within the molecules.
compared with 31P, the radioactive isotope 32P has? ... the atom has more electrons than protons ... Covalent bonds hold atoms together because they. Rating: 4.5 · 2 reviews
Construct SALCs and the molecular orbital diagram for NH\(_3\). This is the first example so far that has more than two pendant atoms and the first example in which the molecule has atoms that lie in three dimensions (ie it is not flat). Ammonia is a trigonal pyramidal molecule, with three pendant hydrogen atoms.
The ammonia molecule in the diagram has the observed bond orientation because ... A. N has four pairs of ... All of the above. E. None of the above.1 answer · 0 votes: D. All of the above.






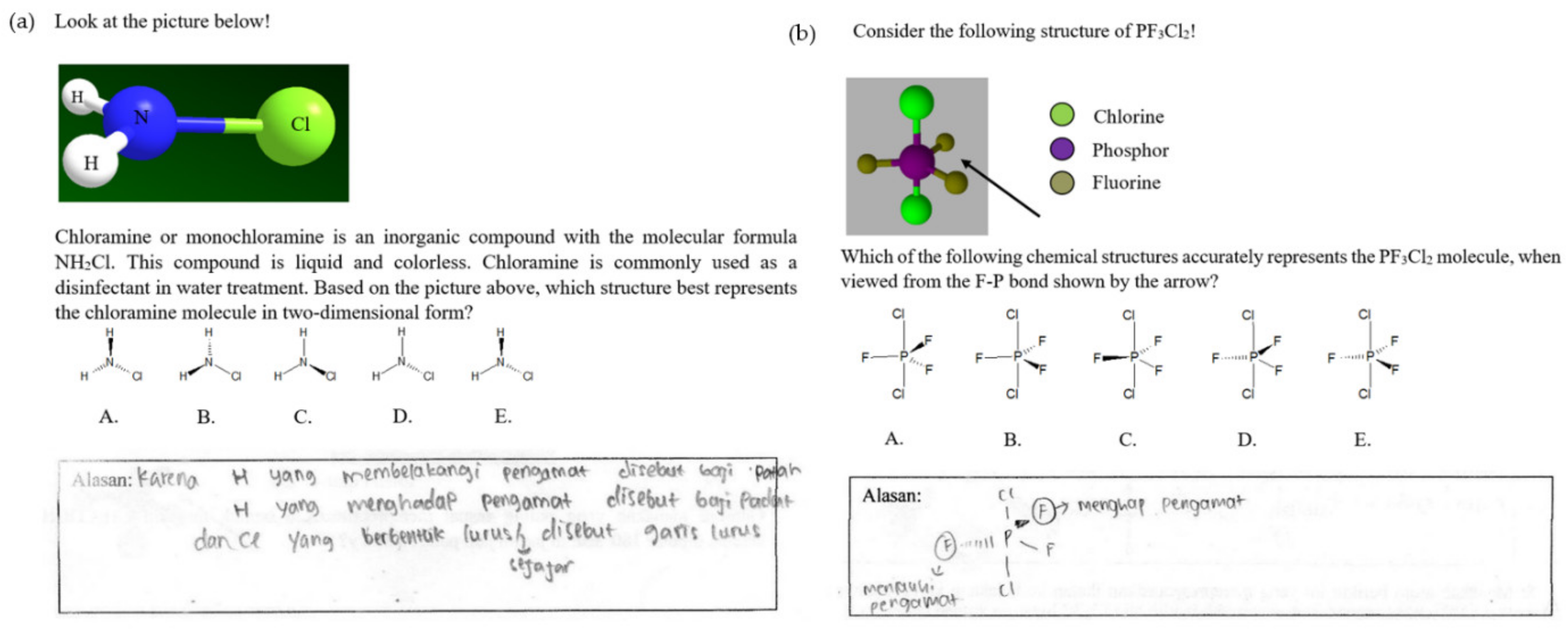












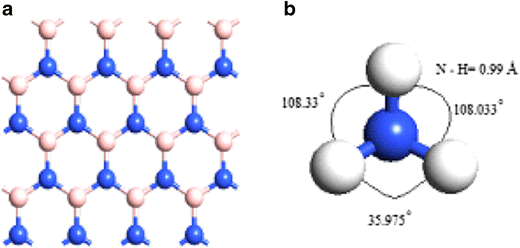

0 Response to "38 the ammonia molecule in the diagram has the observed bond orientation because ..."
Post a Comment