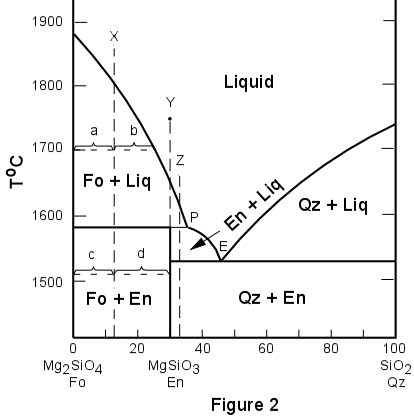
37 degrees of freedom phase diagram
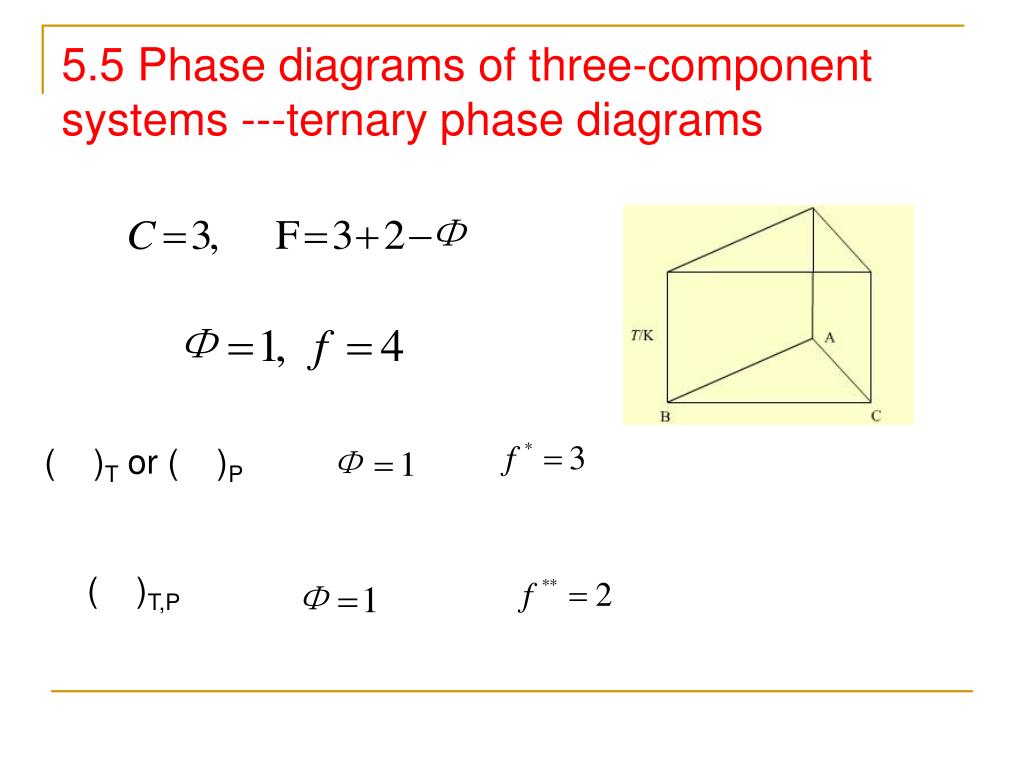
The number 2 in the equation accounts for both temperature and pressure, and implies that they are allowed to change, that is, they are considered degrees of freedom. Consider a hypothetical component for the following unary phase diagram in which the lines divide the solid, liquid, and vapor ... Composition is graphed for a particular, constant Temperature and Pressure condition, so our Degrees of Freedom will be entirely based on ratios of components (i.e. mole fractions). Our ternary diagram has three vertices (S,X,W), and defining any 2 of these defines the last. Hence the phases in the ...
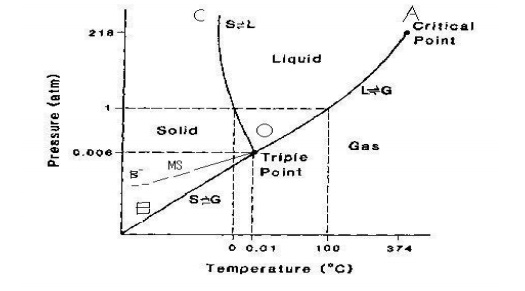
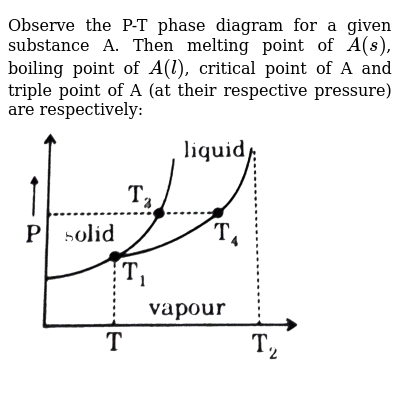
I understand that the number of degrees of freedom is related to other aspects of a thermodynamic system via Gibbs Phase Rule, and I can happily apply that rule, but.... I just don't know what degrees of freedom really *are* in themselves. What does knowing just the variance tell us about a system? How is this used in practice by physical chemists or similar? If I'm interpreting a phase diagram in P-T space of a system with a single component, a point directly on a phase boundary will have ...
Degrees of freedom phase diagram
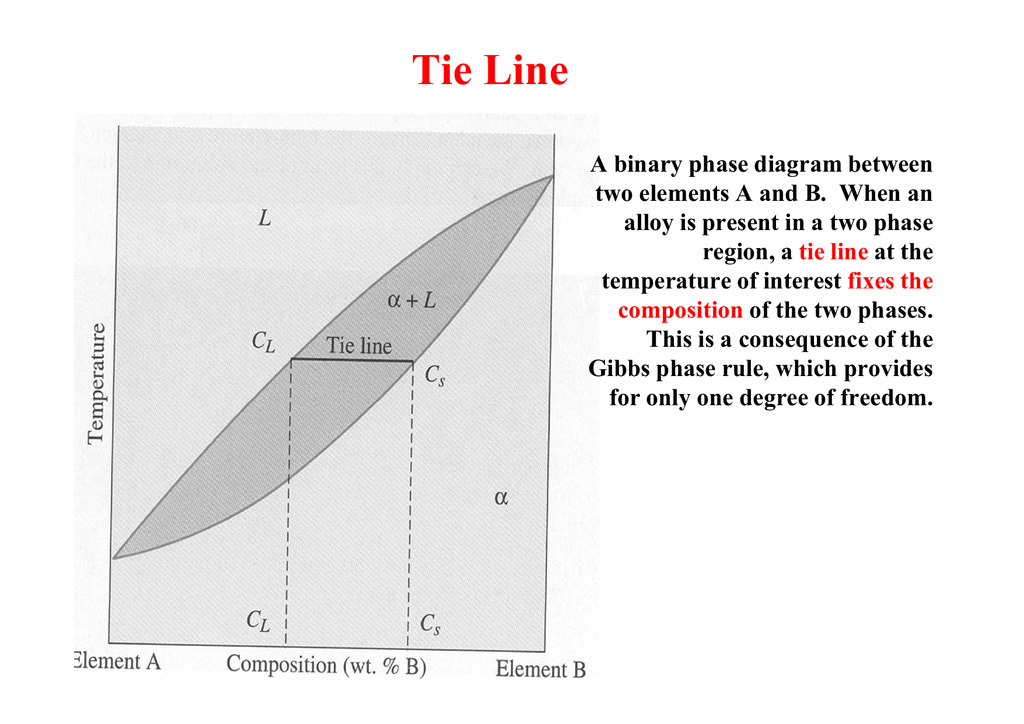
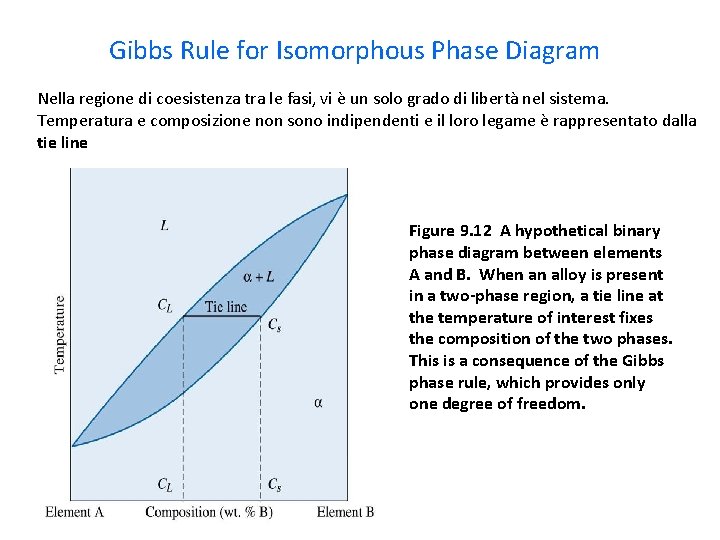
Basically every class I take has what skills you learned throughout it listen in the syllabus. I have one such example here from a class: • Synthesize a block flow diagram from a process description • Identify and modify reaction pathways to achieve specific production while minimizing waste and occurrence of hazardous compounds • Perform a degree of freedom analysis to determine degree of process specification • Perform component, element, and total mass (or molar) material balances • ... July 14, 2020 - If the system point falls within a one-phase area of the phase diagram, the composition variable is the composition of that single phase. There are three degrees of freedom. On the phase diagram, the value of either \(T\) or \(p\) has been fixed, so there are two other independent intensive ... Just looking at that binary phase diagram could have told you all of the above directly. Well, you are wrong. It is obvious, indeed, that there is only one degree of freedom in a two-phase region because we have the splitting into the two composition there. Right.
Degrees of freedom phase diagram. In thermodynamics, the phase rule is a general principle governing "pVT" systems (that is, systems whose states are completely described by the variables pressure (p), volume (V) and temperature (T)) in thermodynamic equilibrium. If F is the number of degrees of freedom, C is the number of ... Within a single-phase field of a temperature-pressure-composition phase diagram, θ, P, and c0 can all be changed independently without changing the number of phases (the number of degrees of freedom is equal to the number of independent thermodynamic fields). IMG here. I've been a member of the sub for a few months now and thought I would write this in case it can help anyone else the way previous such write-ups have helped me :) I started off my Step 1 prep by doing the basic sciences separately before going to FA and UWorld as I was studying for the exam after graduation. **Basic Sciences** *Kaplan* As has been typical for all IMGs, I worked on strengthening my foundations with Kaplan first. I think the only useful material in Kaplan that reall... Note: I am also posting this to r/robotics Hi, I'm looking to plan for the start of my career, so I figured it was the right time to dive into this. I'm entering my final year of university in September (Studying Electrical & Mechanical Engineering), and they've given us a wide variety of options for the courses we can take (I didn't have any choices up until now), so I'm trying to pick the best combination of courses to pursue a career in Robotics/Control. I should note that I engage in a...
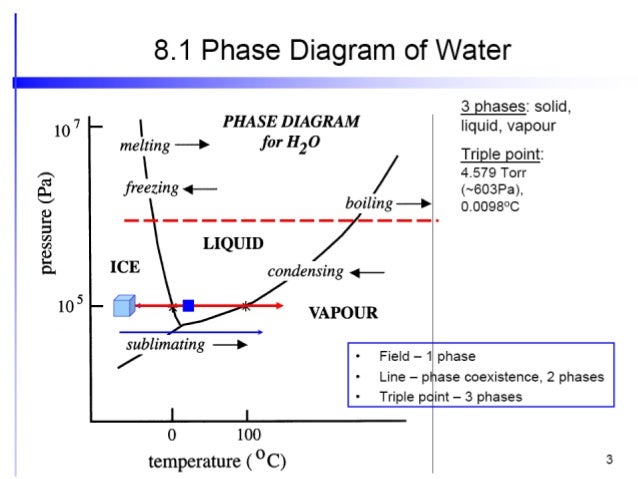
Undoubtedly the main issue with giving players more freedom to conduct space activities is the chronic unrealism that will plague the subreddit as a result, as is the effect when you place teenagers in unilateral control of a national space program. I'm writing this guide to help the mods out, and maybe further convince them that decriminalizing space is a good choice. **Part 1: How to Orbit** Orbiting is literally falling towards the body you're orbiting. Want to get to orbit? Go to the ne... November 14, 2016 - In each of these reactions, either ... is one degree of freedom. In a later section, we will see that the univariant curves represent the condition where ΔGrxn = 0 (i.e. the intersection of the "free energy surface" with the Pressure-Temperature plane represented by the phase diagram)... At this point, it’s beyond the point of being cliché to talk about Star Wars (1977). As ridiculous as what I’m about to say may be, if the following applies to you, I suggest you whole heartedly listen: If you have never seen George Lucas’ 1977 epic space opera Star Wars, I recommend you go and do so now. With that out of the way, I’ll move into what it was I’ve been wishing to discuss. When I first saw Star Wars, as was the case with a great deal of other individuals, I was at an exceedingly y... This shows qualitatively a very simple phase diagram for a given material. The liquid, solid, and gas phases are separated by curves in the T,P parameter space. On these curves, both phases are represented to some degree simultaneously. A slightly more exotic phase transition, this diagram ...
For Complete Courses Download The App Chemistry Untold :- https://play.google.com/store/apps/details?id=co.davos.vcwxy ○Solid State ... April 21, 2012 - The degrees of freedom of a system dictate the number of phases (as described above in the bullet list) that can occur in the system. ... The critical point (on a phase diagram) can only exist at one temperature and pressure for a substance or system and thus the degrees of freedom at any critical ... **Verified?** *(This bot cannot verify AMAs just yet)* **Date:** 2012-09-12 **[Link to submission](http://www.reddit.com/r/IAmA/comments/zrxg1/)** (*Has self-text*) **[Link to my post](http://www.reddit.com/r/IAmA/comments/zrxg1/tabledresser/c67he3b)** Questions|Answers :--|:-- [Why does Facebook constantly make it more difficult for it's users to maintain consistent levels of privacy?](http://www.reddit.com/r/IAmA/comments/zrxg1/iama_facebook_engineer_ama/c676rzc?context=5)|This question ... July 14, 2020 - Starting with the system in this ... same kinds of phases and the same species, but different values of some of the intensive properties. The number of different independent intensive variables that we may change in this way is the number of degrees of freedom or variance, \(F\), ...
Export articles to Mendeley · Get article recommendations from ACS based on references in your Mendeley library
Note: I am also posting this to r/cscareerquestions Hi, I'm looking to plan for the start of my career, so I figured it was the right time to dive into this. I'm entering my final year of university in September (Studying Electrical & Mechanical Engineering), and they've given us a wide variety of options for the courses we can take (I didn't have any choices up until now), so I'm trying to pick the best combination of courses to pursue a career in Robotics/Control. I should note that I eng...
October 21, 2014 - The variance is the number of degrees of freedom we have with regards to things we can change and yet remain within one region on a phase-diagram · where F is the variance, (the number of intensive properties we can vary), C is the number of components, P is the number of phases
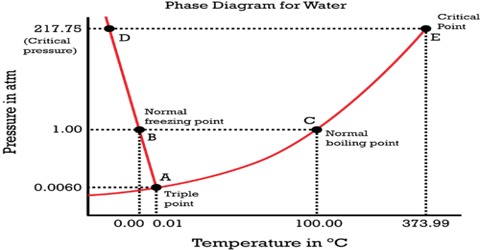
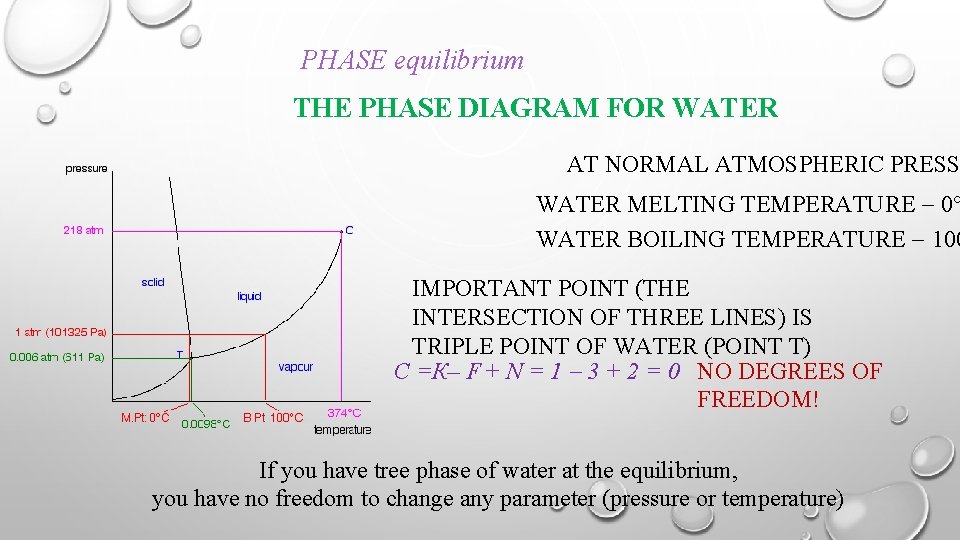
December 1, 2016 - The Gibbs phase rule is $f = c - p + 2$, where $f$ is the degrees of freedom, $c$ is the number of components, and $p$ is the number of phases. Your diagram says $P = \mathrm{cst}$, which I interpret to mean that pressure is constant. That specification lowers the number of degrees of freedom ...

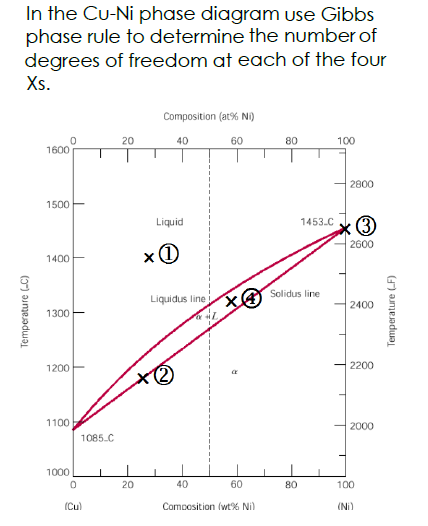
Use Gibbs Phase Rule To Determine The Degrees Of Freedom In A Cu 40 Ni Alloy And Determine The Composition Of Each Phase At The Following Temperatures A 1300 C B 1250 C
The Phase Rule describes the possible number of degrees of freedom in a (closed) system at equilibrium, in terms of the number of separate phases and the number of chemical constituents in the system. It was deduced from thermodynamic principles by J. W. Gibbs in the 1870s.
3 weeks ago - Calculation of salt precipitation and phase diagrams. Extended UNIQUAC software with Microsoft Excel as user interface. Aqueous solutions.
Phase Diagrams of Water & CO2 Explained - Chemistry - Melting, Boiling & Critical Point. The Organic Chemistry Tutor.
Associate Professor, Vice Provost & Associate Vice-President Academic Office: Schmon Tower, 13th floor 905 688 5550 x3528 greg.finn@brocku.caPrecambrian geologist by training, now working in administration, outreach activities and science education. Greg Finn became Vice-Provost and Associate ...
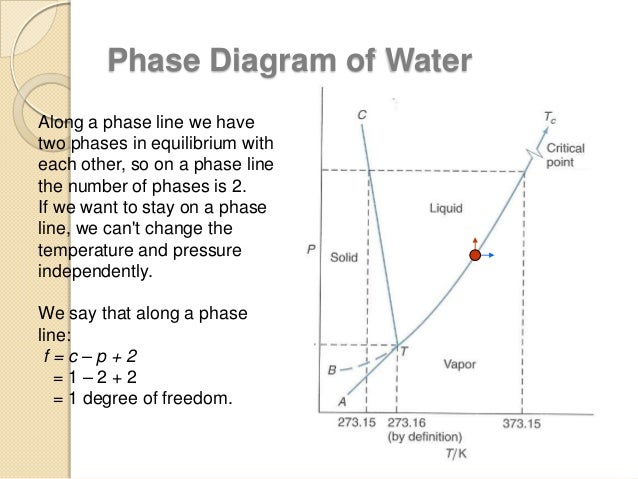
February 7, 2021 - With one component and two ... water vapour above it, in a closed container), there is no degree of freedom, and temperature and pressure are both fixed at what is called the triple point (see phase diagram)....
This proof was given when the papers of Adam Weishaupt’s secret society of "Illuminati" were seized by the Bavarian Government in 1786 and published in 1787. The original blueprint of *world*\-revolution, and the existence of a powerful organization with members in the highest places, were then revealed. From that moment on no doubt remained that all countries and classes of society contained men who were leagued together to destroy *all* legitimate government and *all* religion. The conspirator...
Ppt 5 5 Phase Diagrams Of Three Component Systems Ternary Phase Diagrams Powerpoint Presentation Id 5812150
Gibbs Phase Rule degree of Freedom (example)Subscribe to my channel:https://www.youtube.com/c/ScreenedInstructor?sub_confirmation=1Workbooks ...
Index of /chemistry/PChem/notes
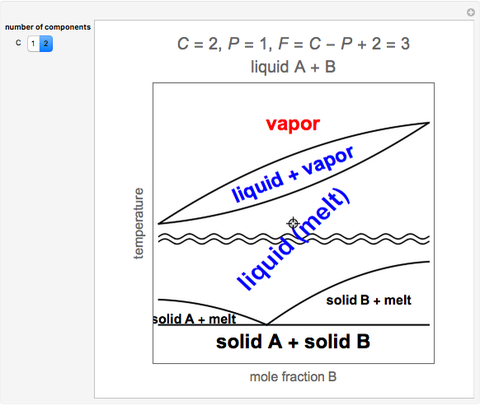
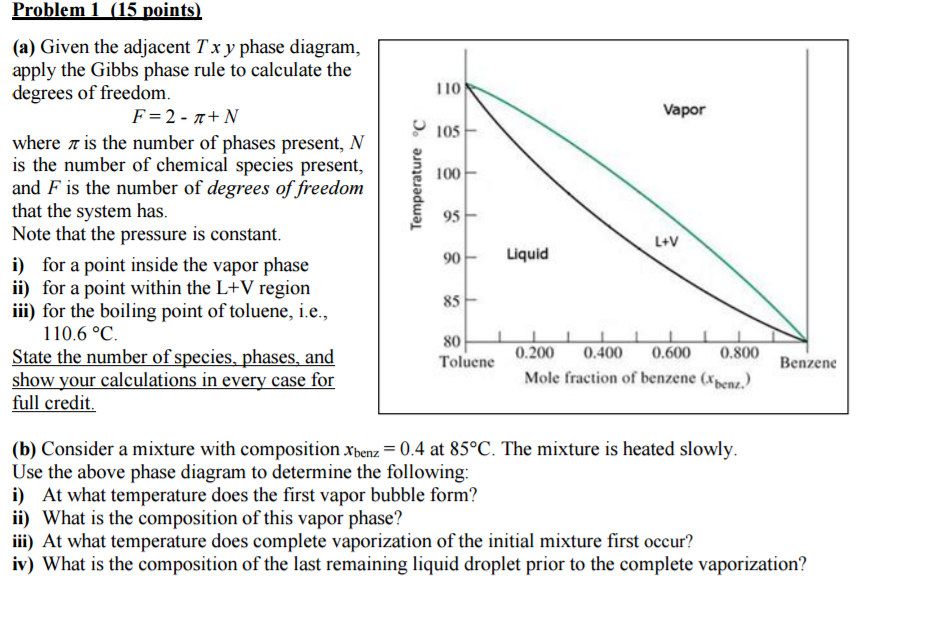
For a binary phase diagram plotted at 1 bar, where composition is displayed on the abscissa and temperature on the ordinate, the number of components c equals two, and the Gibbs phase rule is then f=3−ϕ. In a single-phase region, the number of degree of freedom equals 2, meaning that the ...
Just looking at that binary phase diagram could have told you all of the above directly. Well, you are wrong. It is obvious, indeed, that there is only one degree of freedom in a two-phase region because we have the splitting into the two composition there. Right.
July 14, 2020 - If the system point falls within a one-phase area of the phase diagram, the composition variable is the composition of that single phase. There are three degrees of freedom. On the phase diagram, the value of either \(T\) or \(p\) has been fixed, so there are two other independent intensive ...
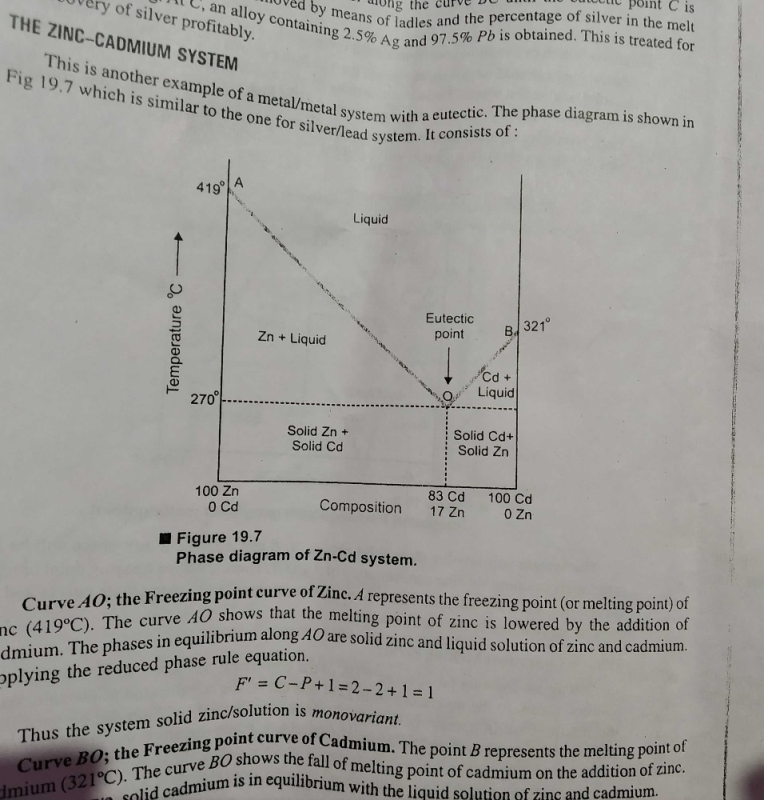
The Number Of Degrees Of Freedom Of Freedom In The Homogeneous Liquid Region Of A Two Component System With A Eutectic Point At One Atmosphere Pressure Is A 0b 1c 2d 3correct Answer Is Option C Can
Basically every class I take has what skills you learned throughout it listen in the syllabus. I have one such example here from a class: • Synthesize a block flow diagram from a process description • Identify and modify reaction pathways to achieve specific production while minimizing waste and occurrence of hazardous compounds • Perform a degree of freedom analysis to determine degree of process specification • Perform component, element, and total mass (or molar) material balances • ...












0 Response to "37 degrees of freedom phase diagram"
Post a Comment