38 energy vs reaction coordinate diagram
*Thermodynamics and Kinetics of Organic Reactions *Electrophiles *Nucleophiles *Organic Reaction Mechanisms in General *Electrophilic Addition *Nucleophilic Substitution *Free Energy of Activation vs Activation Energy *Complex Reaction Coordinate Diagrams *Names and Structures of Organic Molecules *Alkanes *Cycloalkanes *Alkenes This disclosure relates generally to wireless energy transfer, and more specifically to protocol layer coordination of wireless energy transfer ...
Activation energy is the minimum amount of energy it takes to start a chemical reaction. Look at the reaction of hydrogen and oxygen forming water: 2H2 + O2 --> 2H2O.
Energy vs reaction coordinate diagram
The dark reactions use the ATP and NADPH produced during the light reactions for energy, since these reactions do not use any energy from sunlight. The Calvin cycle, which is another name for the ... A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endo the rmic reaction. The fully filled in reaction coordinate diagram is displayed below. This reaction is also exo the rmic because the energy of the products is lower than that of the. February 15, 2013 - Reaction coordinate diagrams are used to relate the free energy changes that occur during the progress of chemical processes to the rate and equilibrium constants of the process. Here I briefly review the application of these diagrams to the thermodynamics ...
Energy vs reaction coordinate diagram. The energy changes that accompany each step of a reaction mechanism are represented on a reaction coordinate diagram. The reaction pathway on this plot is t... Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases. Exothermic reactions release energy to their surroundings, because the products are lower in energy than the reactants. You can think about this visually using a reaction energy diagram, as seen below: And endothermic reaction (left) and an exothermic reaction (right) plotted on a plot of energy against the reaction coordinate (a measure of the ... Energy vs reaction coordinate diagram of an uncatalysed reaction, made by Joe Davies ... Energy vs reaction coordinate diagram of a catalysed (red) ...
An energy level diagram is a great way to compare these two contrasting Systems. Energy level diagram for an Endothermic change. Thermal energy from the Surroundings is absorbed by the System. Sie scheinen keine Seiten mit Frames betrachten zu können - klicken Sie hier zur Umgehung des Frames · Your browser seems not to support frames - click here to circumvent the frame Description of the Reaction Coordinate - YouTube · AboutPressCopyrightContact usCreatorsAdvertiseDevelopersTermsPrivacyPolicy & SafetyHow YouTube worksTest new features · © 2021 Google LLC Some of the worksheet s for this concept are Potential energy diagram work answers, Work 1 2 potential energy diagram s key, Ws 4 potential energy diagram s work, Name kinetics potential energy diagram s, Work 1 2 potential energy diagram s, Chemistry 12 work 1 2, Energy diagram s, Forms of energy lesson plan chemical energy. Unit 1-Reaction ...
The energy vs. reaction coordinate graph shows an exothermic reaction where the products have lower energy than the reactants. While the product is lower energy than the reactant, there is still a ... Figure \(\PageIndex{1}\): Energy profile diagram for A → B (left) and A → C (right). The horizontal axis is a reaction coordinate, and the vertical axis represents Gibbs energy. The delocalized carbocation intermediate (A) is the protonated form of 1,3-butadiene (first step of the reaction of 1,3-butadiene with HBr). Energy Diagram – Energy Graph – Energy Chart – Energy diagrams – energy graphs – energy charts – energy diagram ... For a chemical reaction or process an energy profile (or reaction coordinate diagram) is a theoretical representation of a single energetic pathway, along the reaction coordinate, as the reactants are transformed into products. Reaction coordinate diagrams are derived from the corresponding ...
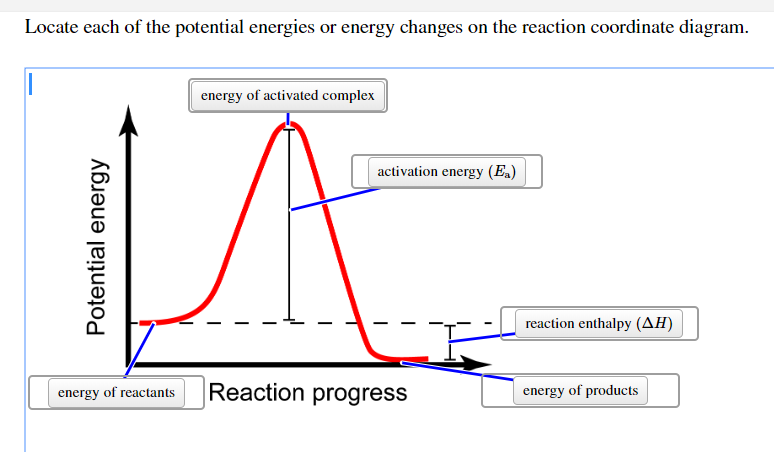
Reaction Coordinate Diagrams Let's consider a general reaction where a reactant or set of reactants, A, is transformed into a product or set of products, B. The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction.
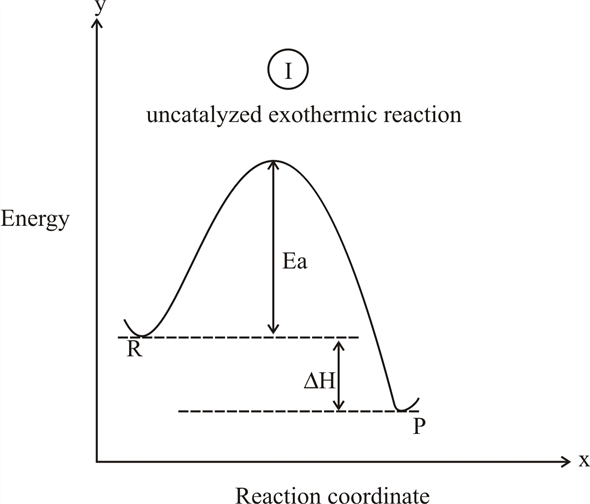
The fully filled in reaction coordinate diagram is displayed below. The arrow marked in the question represents the activation energy, which is the energy barrier that must be overcome in order for the reactants to form products.
... Reactions arrow movements ask questions axial position ballroom dancing Biochemistry Biology Biomolecules Bond Forming bonus opportunities bonus ...
Re: Circular Standing Electron Diagram. Postby Nathan Sigel 2k » Sun Oct 10, 2021 10:28 pm. Yeah, pretty much everything you said is correct. To be in a particular energy level, the electron in that orbital must oscillate only a certain number of times (whole number). There has to be a set number of full wave cycles for the electron to be in a ...
Solved The Reaction Coordinate Diagram Below Is For An Uncatalyzed Chemical Reaction Answer The Following Questions A What Overall Conclusion Ab Course Hero
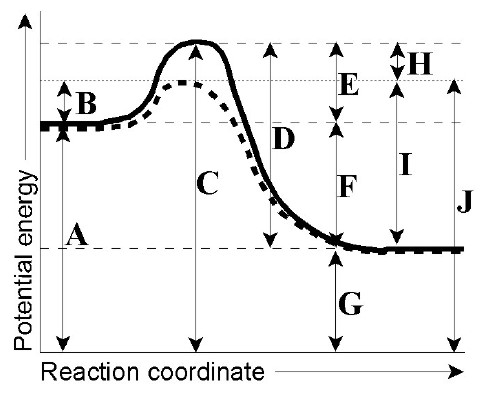
I am confused on whether in a reaction coordinate diagram, does the difference between the reactants and products equal deltaH or deltaG I have seen graphs that demonstrate both. If some could explain that would be great!   for reference this khan article states it as deltaH: https://www.khanacademy.org/test-prep/mcat/chemical-processes/thermochemistry/a/endothermic-vs-exothermic-reactions   This khan article states it as deltaG: https://www.khanacademy.org/science/biolog...

May The Reaction Coordinate In A Chemical Reaction Coordinate Diagram Be Represented By A Time Axis Physics Stack Exchange
Note: I take Pure Chem + Bio. All Physics tips are courtesy of my classmates and this community ([https://www.reddit.com/r/SGExams/comments/qqr4pn/o\_levels\_pure\_physicscombined\_science\_p1\_tips/](https://www.reddit.com/r/SGExams/comments/qqr4pn/o_levels_pure_physicscombined_science_p1_tips/)) ​ 3rd last exam day till the end. Might be your last day for some. Either way, hope you had a good rest yesterday. Pure Physics starts at 8am, so make sure to head to school earlier. &a...
In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ‘reaction coordinate’, tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical SN2 reaction ...
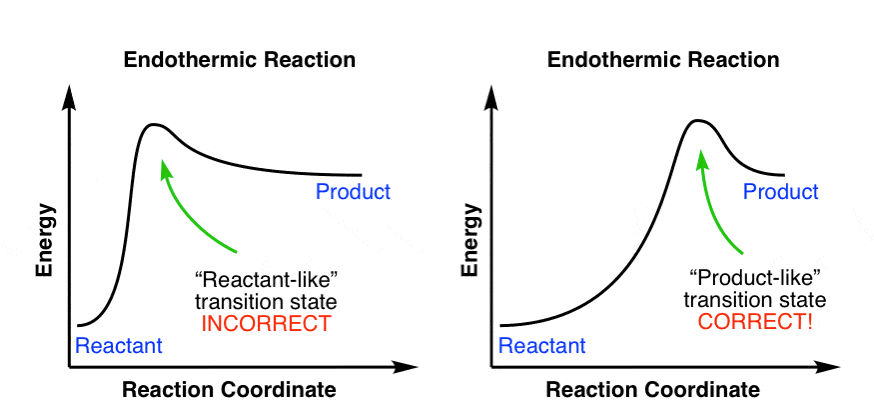
A reaction will be exothermic if the energy of the products is less than the energy of the reactants. A reaction is endothermic when the energy of the products is greater than the energy of the reactants. The is for an exothermic reaction. Below is a reaction coordinate diagram for an endothermic ...
Which diagram correctly depicts the general trend in first ionization energy? which diagram correctly depicts the general trend in first ionization energy? b. which diagram correctly depicts the trend in electronegativity? b. which is defined as the energy required to remove an electron from an atom of an element in the gaseous state ...
a The formula diagrams. b LSV polarization ... DFT calculations have been subsequently conducted to reveal the detailed free energy profiles for each reaction coordinate during ECR-to-CH 4 ...
... Reliability Petrochemicals Process Control Process Optimization Project Management Refining Home 2020 April 2020 Piping and instrument diagrams (P ...
September 12, 2021 - In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ‘reaction coordinate’, tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction ...
Compute energy and gradient using : ... Energy vs. ... reaction coordinate plot.
Time-saving chemistry videos explaining energy diagrams. Energy diagrams are graphs which depict the progress of a reaction v. it's energy. They are useful to understand the change in enthalpy of a reaction and the activation energy.
We ’ ll go into more detail on the reversibility of the Diels-Alder (and kinetic vs. ... energy of the electrons (along with a corresponding ...
e, f The potential energy curve vs. reaction coordinate for MA/FA desorption on the surface of MAPbI 3 /FAPbI 3 with iodide vacancies (e) and on the surfaces of Cs 0.125 FA 0.875 PbI 3 (12.5% Cs ...
b A traditional reaction coordinate diagram used to represent both the free energies of reaction, ΔΦ, and activation energies, E a. This is generalized by a weighted, directed graph connecting ...
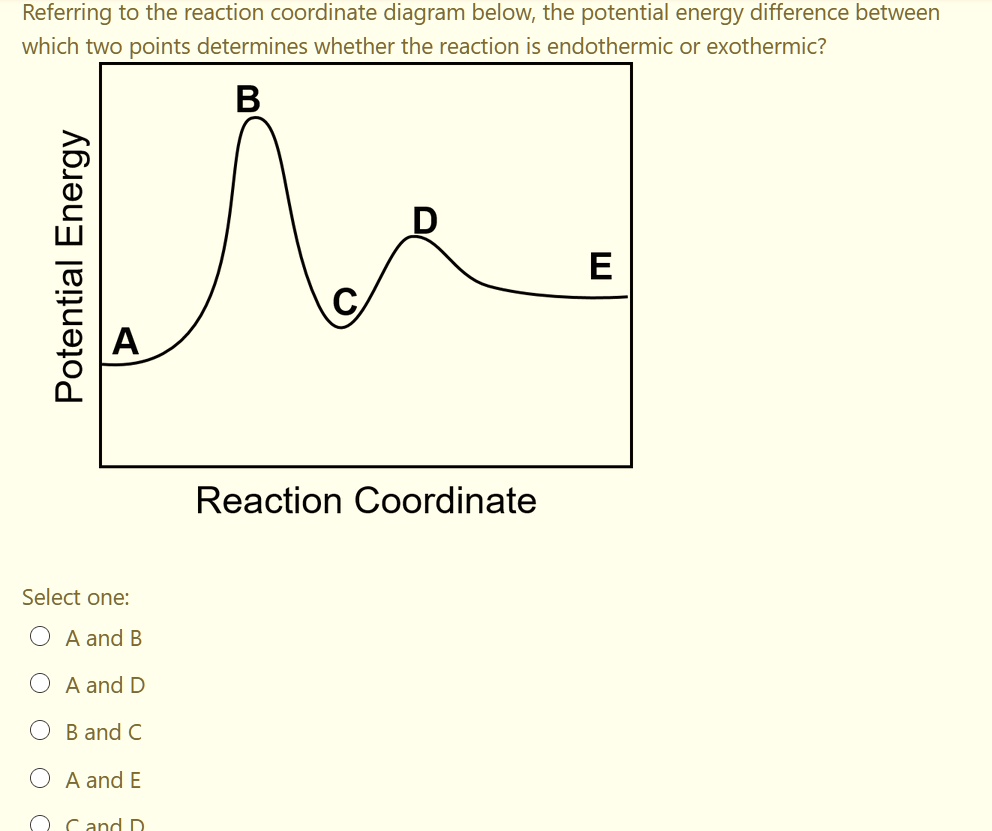
Solved Referring To The Reaction Coordinate Diagram Below The Potential Energy Difference Between Which Two Points Determines Whether The Reaction Is Endothermic Or Exothermic E 1 A E Reaction Coordinate Select One A
At −0.50 V vs. RHE when the reaction ... b Free energy diagram showing ... The intermediates and their energy profile across the reaction coordinate are displayed in the free energy ...
I want to investigated the potential energy diagram for HXeXeF molecule in singlet state and triplet state (at the singlet state geometry) along the H-Xe coordinate to obtain crossing point of them.
Hey everyone, I’m Bill and I’m currently in my 2nd year of a BBiomed single degree here at Monash. A few months ago, I’ve put up an expression of interest for a biomed + chem study skills post à la allevana and luneax, but man, I’ve been swamped with uni work for the past 9 weeks (thank you very much Monash for that 3/4 sem break /s). I’ll write a quick paragraph on every single subject I did, but mainly focusing on pitfalls to beware of because these are probably the most valuable for future st...
Another way describe a reaction mechanism is to plot the change in energy along the reaction pathway. This is shown in an Energy vs Reaction Coordinate plot. In these plots, the y-axis is energy (with lower E being more stable). The x-axis is the reaction coordinate, which is a plot of the ...
a) Rate " k[CH3CHO] (Products are CH3 and CHO.) (b) Rate " k[I]2[Ar] (Products are I2 and Ar; the role of the Ar is to remove energy as the ...
Reaction rate (a) and Faradaic efficiency (b) for NORR on a Pt foil, Cu Foil, and Cu foam for 600 s, and NRR over Cu foam for 2 h at -0.9 V vs. RHE. (c) Linear Sweep Voltammetry (LSV) for Pt foil, Cu foil and Cu foam in NO-saturated 0.25 mol/L Li 2 SO 4 Cu foam in Ar or N 2-saturated 0.25 mol/L Li 2 SO 4 acquired with a scan rate of 10 mV/s at ...
... 9-15 (A) Transition state diagram illustrating Gibbs energy vs reaction coordinate for conversion of reactants to products in a chemical reaction ...
Figure 1: Reaction coordinate diagram for the bimolecular nucleophilic substitution (S N 2) reaction between bromomethane and the hydroxide anion. ... (now referred to as activation energy) of a reaction is equal to the average energy of all molecules undergoing reaction minus the average energy of all reactant molecules.
Reaction potential energy diagrams are graphs that show the energy of a process as a function of the extent to which that process has occurred. As these are graphs showing mathematical functions, …
June 22, 2020 - Reaction Energy Profiles
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation

May The Reaction Coordinate In A Chemical Reaction Coordinate Diagram Be Represented By A Time Axis Physics Stack Exchange
... connect discrete (loss of leaving group, formation of carbocation, formation of pi-bond) to energy maxima/minima in the reaction coordinate diagram.
Professor Patricia Shapley, University of Illinois, 2012
Unlike Figure 2a), the energy profile diagram Figure 2b contains an abscissa. It represents the reaction coordinate that concerns changes of nuclear geometry during the reaction. It corresponds to the usual energy diagrams used in teaching at high school and in undergraduate courses at university.
Arizona State University in Tempe, AZ is a public research university ranked #1 in the U.S. for innovation, dedicated to accessibility and academic excellence.
Covalent Modification Vs Allosteric Regulation ... covalent modifications to enzymes but first lets review the idea that enzymes make reactions go faster and looking at a reaction coordinate diagram youd notice that enzymes do this by lowering the reactions activation energy also before we talk about covalently modified enzymes I want to remind ...
Most of the time, chemical energy is released in the form of heat, and this transformation from chemical energy to heat, or thermal energy, is called an exothermic reaction. Next, there are two ...

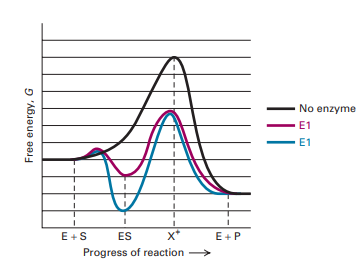
Energy Diagrams For Enzyme Catalyzed Reactions Concepts And Misconcepts Aledo 2003 Biochemistry And Molecular Biology Education Wiley Online Library
13.10.2021 · Converting intermittent renewable energy resources, such as solar and wind energy, into storable chemical fuels is a key pathway toward a sustainable …This potential energy diagram shows the effect of a catalyst on the activation energy.The catalyst provides a different reaction path with a lower activation energy.As shown, the catalyzed pathway involves a two-step mechanism ...
In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ‘reaction coordinate’, tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical SN2 reaction ...
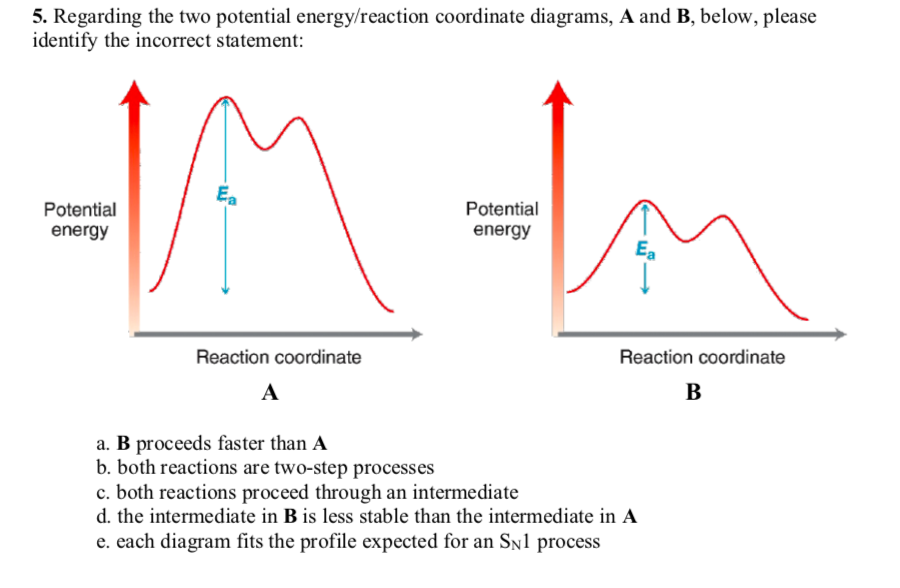
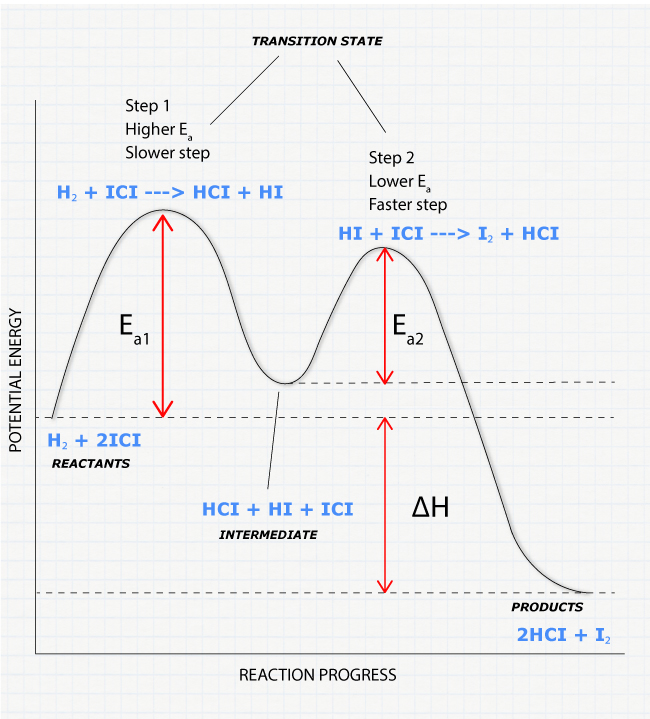
(4) A. Write "INT" at the position of any intermediates and "TS" at the position of any transition states in the reaction coordinate diagram free energy kJ/mol Review reaction coordinate 1 answer Help Please 8.
In the formalism of transition-state ... state to the configuration of the products. The reaction coordinate is typically chosen to follow the path along the gradient (path of shallowest ascent/deepest descent) of potential energy from reactants to products....
















0 Response to "38 energy vs reaction coordinate diagram"
Post a Comment