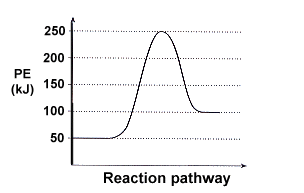
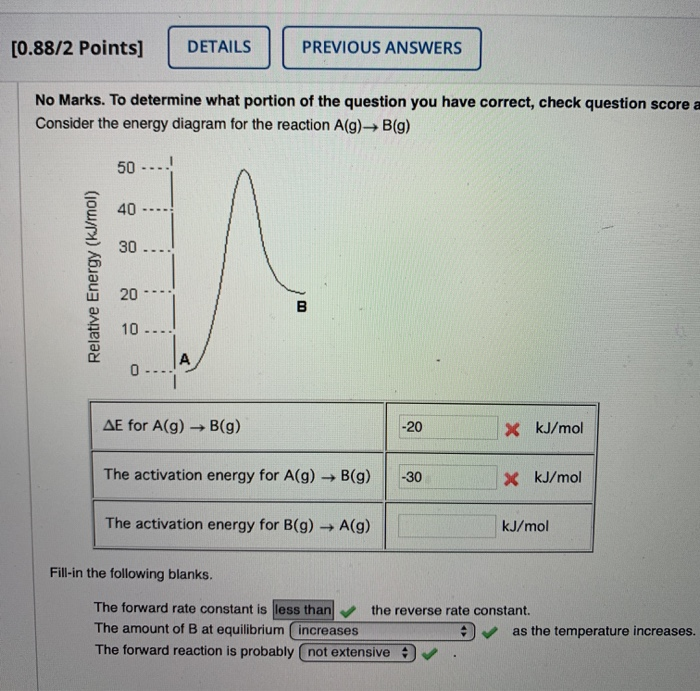
39 consider the energy diagram for the reaction a(g) b(g)
(b) Ecell and A, G of cell reaction both are intensive properties (c) Ecell is an intensive property while A.G of cell reaction is an extensive property ... Consider the following diagram and answer the questions Pe 6) to (vi) given below. ... chemical energy to electrical energy. A. True B. False: 12: 404: Consider the table of standard ... Consider the energy diagram below. A graph with Reaction Progression on the x axis and Energy on the y axis. A line starts low on the y axis; this section runs horizontally and is labeled A. It rises sharply to a high round peak labeled C.
Representing a Reaction with a Potential Energy Diagram ... CH4(g). b. Draw a potential energy diagram that would reasonably represent this combustion.

Consider the energy diagram for the reaction a(g) b(g)
Consider the energy diagram for the reaction A(g) B(g) E for A(g) B(g) (No Response) kJ/mol The activation energy for A(g) B(g) (No ... The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy ... Base your answers to questions 24 --- 26 on the potential energy diagram shown ... What is the overall result when CH4(g) burns according to this reaction?
Consider the energy diagram for the reaction a(g) b(g). The stable form is the one with the lowest Gibbs free energy. At 300 K, the enthalpy difference between diamond and graphite is Ahd-g = 1900 J/mole, with diamond less stable than graphite in this regard. Being a highly ordered structure, diamond has a molar entropy lower than that of graphite, and Asd-g = -3.3 J/mole-K (see Fig. 3.6). Assertion (A) : In a reaction of copper with oxygen,copper serves as a reducing agent. Reason (R) : The substance which gains oxygen in a chemical reaction is a reducing agents. 306. Assertion : The following chemical equation, 2C H O CO H O 2. 7 66 2 + + $4 3 2 2. is a balanced chemical equation. At 298 K, a reaction with ΔG ‡ = 23 kcal/mol has a rate constant of k ≈ 8.4 × 10 -5 s -1 and a half life of t 1/2 ≈ 2.3 hours, figures that are often rounded to k ~ 10 -4 s -1 and t 1/2 ~ 2 h. Thus, a free energy of activation of this magnitude corresponds to a typical reaction that proceeds to completion overnight at room ... Fill-in the following blanks. The forward rate constant is ---Select--- greater than about the same as less than the reverse rate constant. The amount of B ...
Consider the reaction X + Y → Z The following data are obtained at 360 K Determine the rate law and the overall order of the reaction. Show all steps clearly below and in the table above. b) Determine the rate of reaction for "experiment 6", where [X]initial = 0.50 M and [Y]initial= 0.20 M. Show all work and units. 1 Answer to single-displacement reactions Complete and balance each of the following single-displacement reactions. Include physical states. Consider the following molecular-level diagram of a chemical reaction between N2(g) and Cl2(g) . The small blue spheres represent nitrogen atoms and... Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams. Gibbs Free Energy. Q. 2CO (g)+2NO (g)→2CO2 (g)+N2 (g). Use standard free energies of formation to determine ΔG∘rxn for this reaction at 25°C. Solved • Apr 7, 2021. Gibbs Free Energy. Q. For each of the following reactions, calculate ΔH°rxn, ΔS°rxn, and ΔG°rxn at 25 °C. State whether or not the reaction is spontaneous.
Ping, Y., Nielsen, R. J. & Goddard, W. A. III The reaction mechanism with free energy barriers at constant potentials for the oxygen evolution reaction at the IrO 2 (110) surface. J. Am. Consider the above mentioned two chemical equations with two different kinds of arrows (- and . ) along ... It is double displacement reaction. b. H2 (g) +. Cl2 (g) sunlight 2HCl(g) ... (g) $ 2CuO(s) In a schematic diagram for the preparation of hydrogen could be. A) C2H6(g) B) CO2(g). (C) HICE). D) NH3(2). 7. The potential energy diagram for a chemical reaction is shown below. Reaction Coordinate. Consider the following reaction: CH4 +2O2 -> CO2 + 2H2O. Delta H = -891 kJ Calculate the enthalpy change for each of the following cases: a. 1.00 g methane is burned in excess oxygen b. 1.00 x 10^3 L methane gas at 740. torr and.
Damped harmonic oscillators are vibrating systems for which the amplitude of vibration decreases over time. Since nearly all physical systems involve considerations such as air resistance, friction, and intermolecular forces where energy in the system is lost to heat or sound, accounting for damping is important in realistic oscillatory systems. Examples of damped harmonic oscillators include ...
A scientist measures the standard enthalpy change for the following reaction to be -171.2 kJ: 2SO2(g) + O2(g) 2SO3(g) Based on this value and the standard enthalpies of formation for the other subst

Solved Figure 16 21 Is An Energy Level Diagram For A Reaction Match The Appropriate Number With The Quantity It Represents Begin Equation Begin Array L Text A Reactants Text B Activated Complex
Transcribed image text: Consider the energy diagram for the reaction A(g) rightarrow B(g) Delta E for A(g) rightarrow B(g) 30 kJ/mol The activation energy ...
(a) Helmholtz Free Energy (A) (b) Internal Energy (E) (c) Work Function (d) Gibbs Free Energy. Answer. Answer: (d) Gibbs Free Energy Explanation: ∆G = ∆H - T∆S ΔG predicts the direction in which a chemical reaction will go under two conditions: (1) constant temperature and (2) constant pressure.
7. Three beakers labelled as A, B and C each containing 25 ml of water were taken. A small amount of NaOH, anhydrous CuSO 4 and NaCl were added to the beakers A, B and C respectively. It was observed that there was an increase in the temperature of the solution contained in beakers A and B, whereas in case of beaker C, the temperature of the solution falls.
One of the early theories proposed by researchers was known as the James-Lange theory of emotion. 1 . Proposed independently by psychologist William James and physiologist Carl Lange, the James-Lange theory of emotion suggested that emotions occur as a result of physiological reactions to events. In other words, this theory proposes that ...
Ethane, a minority component of natural gas, burns to form carbon dioxide and water according to this reaction:2H3C−CH3(g)+7O2(g)→4CO2(g)+6H2O(g) Note that the average bond energy for the breaking of a bond in CO2 is 799 kJ/mol.
2.00 g of Benzoic acid are burned in a bomb calorimeter, and the temperature increases from 17.84 degrees C to 23.34 degrees C. Calculate the Gibbs energy of reaction for the combustion of 4.08 g Arg
Standard Gibbs Free energy change ( Delta G^{o} ) for a reaction is zero. The value of equilibrium constant of the reaction will be: ... Consider the below diagram of heat ... For the reaction 2A(g) + B(g) — 2D(g) AUⓇ = - 10.5 k) and AS = - 44.1 k-1 ...
What Is The Activation Energy For The Reverse Reaction In Terms Of The Activation Energy Of The Forward Reaction And The Enthalpy Of Reaction Draw It Out For An Endothermic Reaction
CBSE Sample Papers for Class 10 Science Set 1 with Solutions. (i) The question paper comprises four sections A, B, C and D. There are 36 questions in the question paper. All questions are compulsory. (ii) Section-A - question no. 1 to 20 - all questions and parts there of are of one mark each.

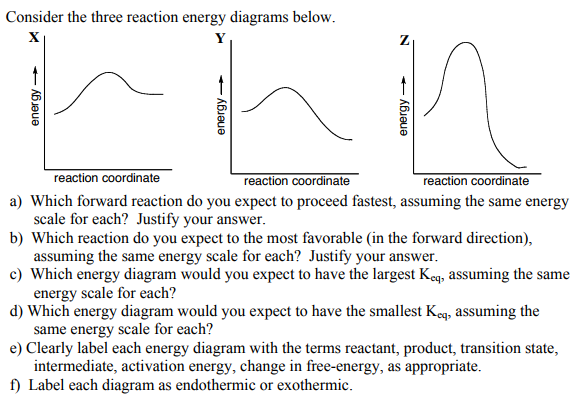
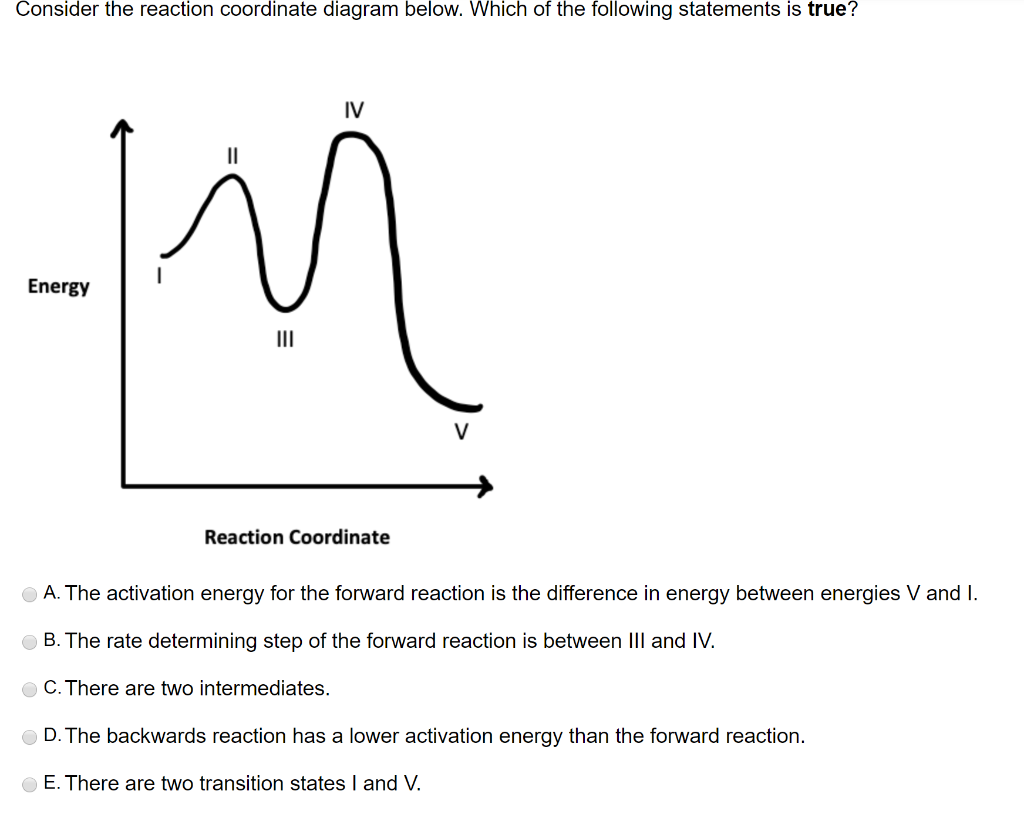
3 Consider The Following Four Energy Diagrams Rxn A Which Diagrams Correspond With A Two Step Mechanism Homeworklib
Consider the energy diagram for the reaction A(g) B(g) E for A(g) B(g) 30 kJ/mol The activation energy for A(g) B(g) 20 kJ/mol The activation energy for ...
Hey guys I'm struggling, the reaction given is 2CO+O2->2CO2 It says current monoxide and oxygen combine to produce carbon dioxide. The total bond energy of the products is 1472kJ. The bond energy of each carbon oxygen bond in carbon dioxide it is____. Is it 368kJ 2,944kJ 736kJ Or 1,472kJ Can someone also please explain how to do this thank you!
7O49, 7O4A, 7O4B, 7O4C, 7OK9. PubMed Abstract: The penicillin-binding proteins are the enzyme catalysts of the critical transpeptidation crosslinking polymerization reaction of bacterial peptidoglycan synthesis and the molecular targets of the penicillin antibiotics. Here, we report a combined crystallographic, small-angle X-ray scattering ...
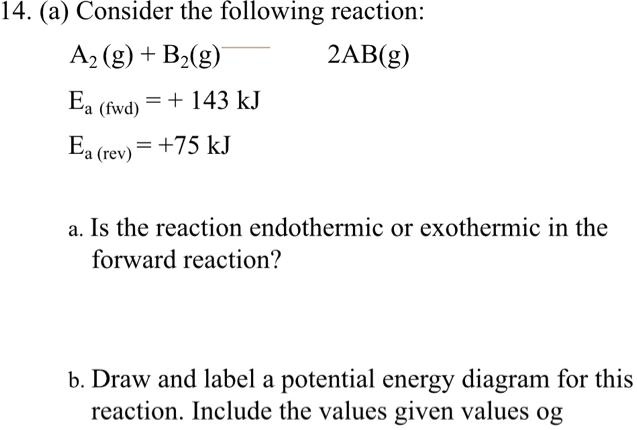
Solved 14 A Consider The Following Reaction Az G Bz G 2ab G Ea Fwvd 143 Kj Ea Rev 75 Kj Is The Reaction Endothermic Or Exothermic In The Forward Reaction
Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
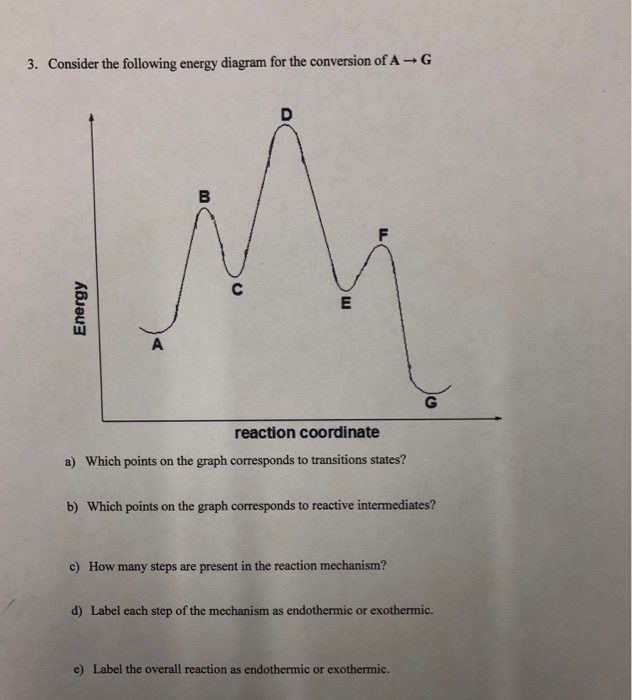
4. Consider the potential energy diagram for the reaction: AB+C longrightarrow A+BC. a.) Potential energy of the reactants: b.) Potential ...
Fig. 4(a, b) is the XRD patterns of PANI/G and G.For pure PANI, it can be seen three diffraction peaks, which are caused by periodic parallelism and arrangement of PANI chains. With the increase of the mass fraction of G, the diffraction peak becomes sharper, indicating the successful synthesis of PANI/G composite material.
Answer: D. Exothermic. Explanation: Hello there! In this case, since the potential energy versus reaction progress diagrams are related to the energetic profile of a chemical reaction, we can set the initial point at the beginning of the reaction as the energy of the reactants and the final point as the energy of the products.
C(s) + O 2 (g) → CO 2 (g) ΔH = -394 kJ When 1 mole of carbon burns completely in oxygen to form carbon dioxide, 394 kJ of heat is released. The heat of combustion of carbon is -394 kJ mol-1. The energy level diagram for the combustion of carbon is as shown below. (b) Combustion of compounds CH 4 (g) + O 2 (g) → CO 2 (g) + 2H 2 O(l)

6 26 Consider The Folloving Four Energy Diagrams Potential Energy Potential Energy Reaction Coordinate Reaction Coordinate Potential Homeworklib
What is the sign of the enthalpy change for an endothermic reaction? exothermic? How many joules of heat are required to heat 15.00 g of lead from 25°C to ...

2 Consider The Reaction Energy Diagram Below Assuming That Both The Reactions Are Reversible List All Homeworklib
(b) Compute the heat generated while transferring 9600 coulombs of charge in two hours through a potential difference of 40 V. Ans. (a) The mathematical expression for Joul's law of heating is H = i²Rt, where H-heat energy, R- resistance in the circuit, t- time of current stayed in the circuit.
b) Total strain multiplied by the volume of the member. c) The maximum strain multiplied by the length of the member. d) Product of strain and Young's modulus of the material. 19.The ability of a material to absorb energy when elastically deformed and to return it when unloaded is called __________. a) Elasticity.
Base your answers to questions 24 --- 26 on the potential energy diagram shown ... What is the overall result when CH4(g) burns according to this reaction?
The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy ...
Consider the energy diagram for the reaction A(g) B(g) E for A(g) B(g) (No Response) kJ/mol The activation energy for A(g) B(g) (No ...
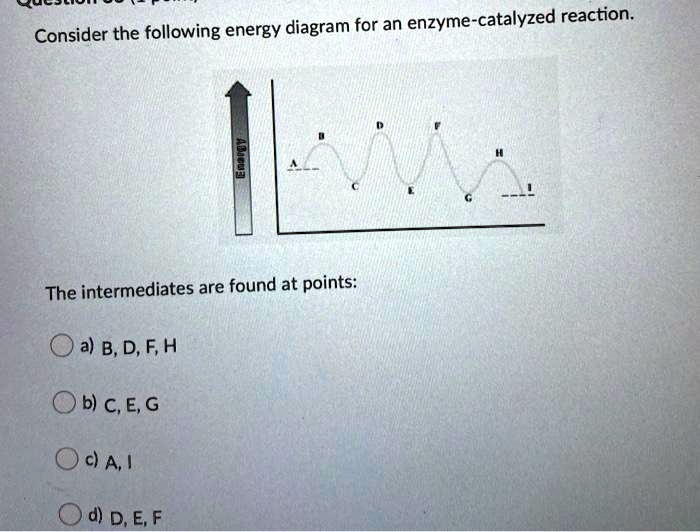
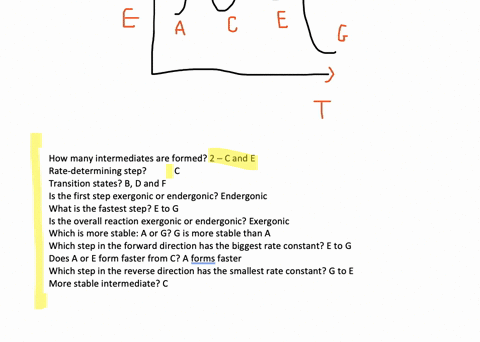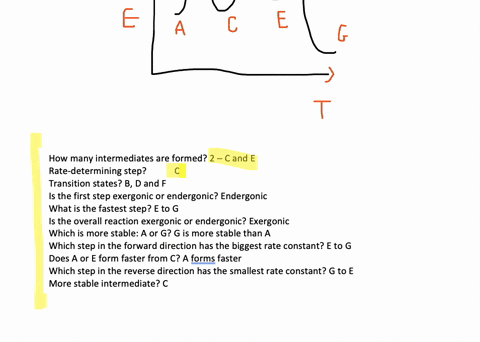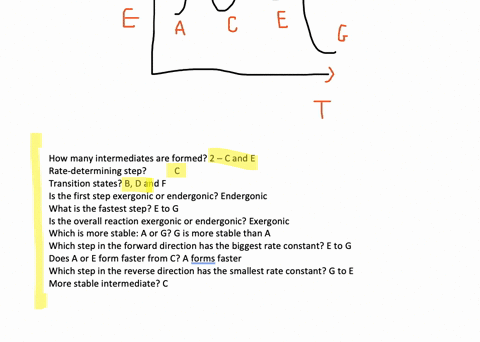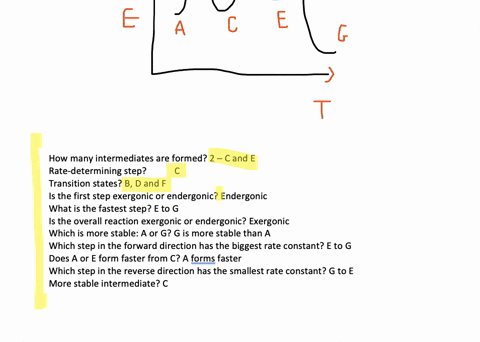
Solved Consider The Following Energy Diagram For An Enzyme Catalyzed Reaction The Intermediates Are Found At Points Oa B D F H B C E G Oda D D E F
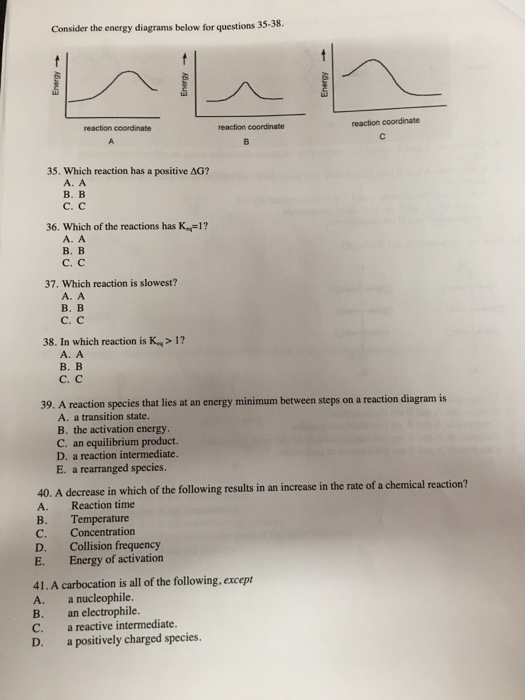
Solved 15 Refer To The Reaction Energy Diagrams Below Left And Right To Answer Questions A C 2pts Each Left Right A Which Reaction Energy Diagram Represents Reaction In Which Keq Is Less
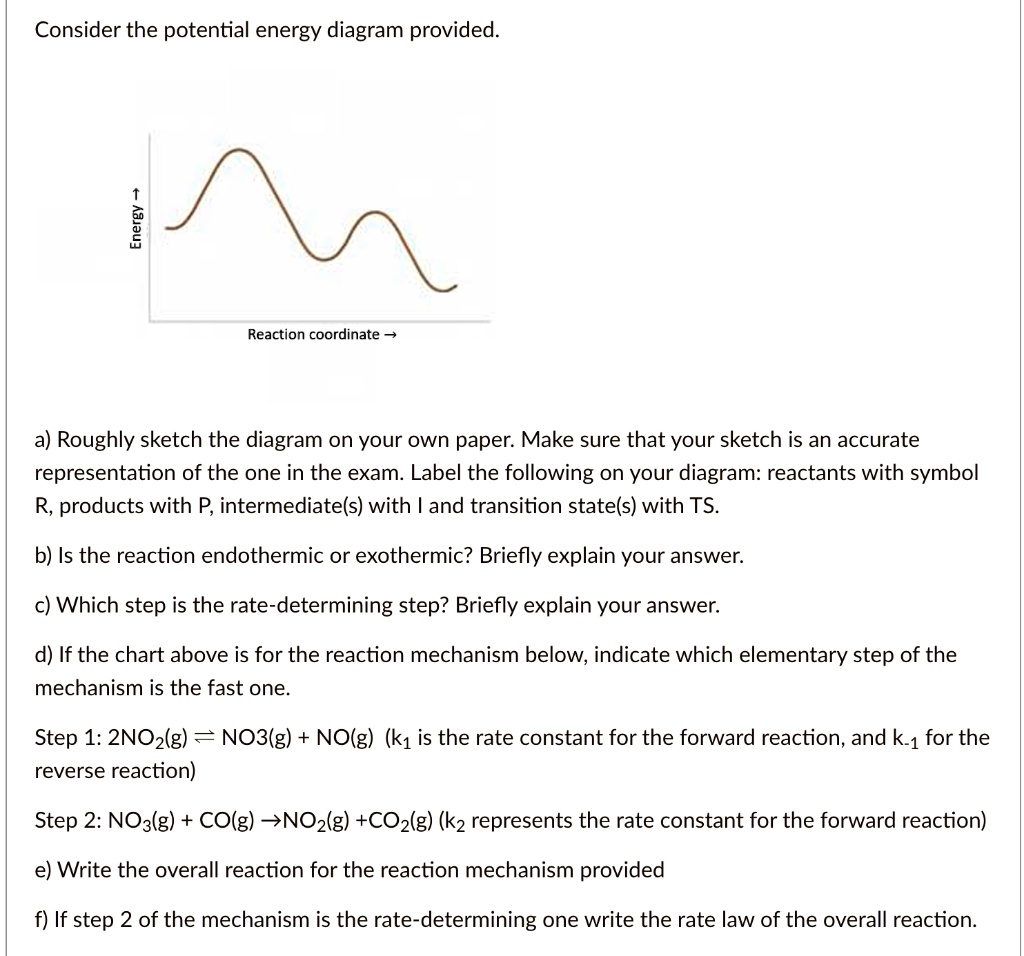
Solved Consider The Potential Energy Diagram Provided 1 Reaction Coordinate A Roughly Sketch The Diagram On Your Own Paper Make Sure That Your Sketch Is An Accurate Representation Of The One In The
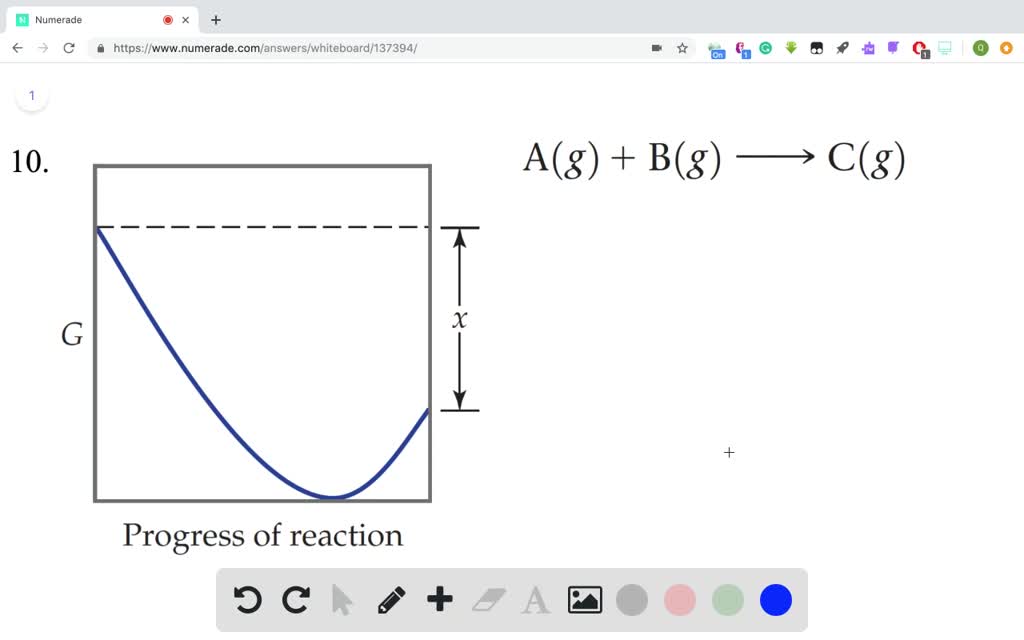
Solved The Accompanying Diagram Shows How The Free Energy G Changes During A Hypothetical Reaction A G B G Longrightarrow C G On The Left Are Pure Reactants A And Mathrm B Each At 1 Mathrm Atm And








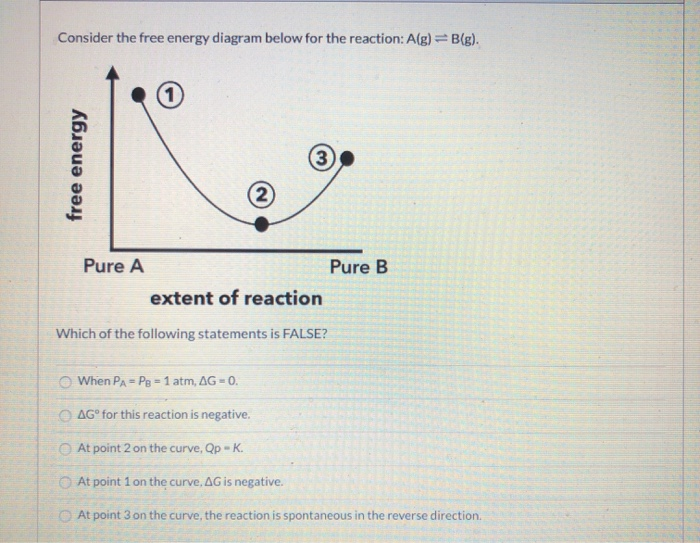
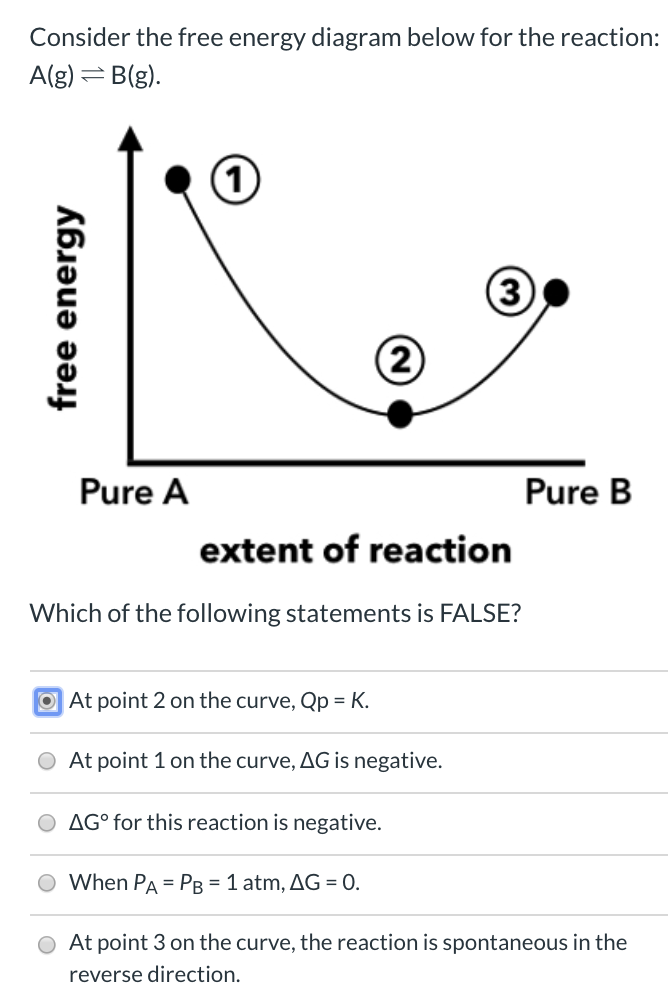



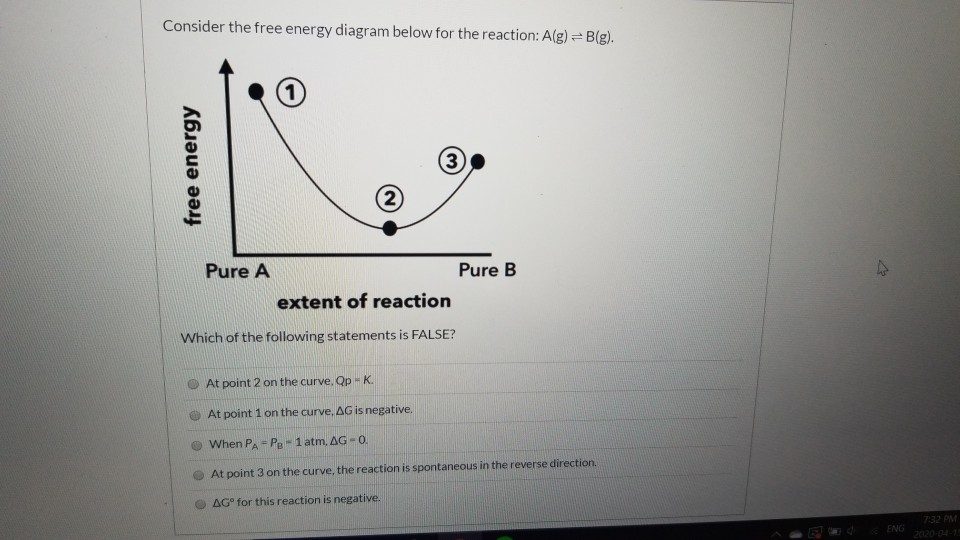
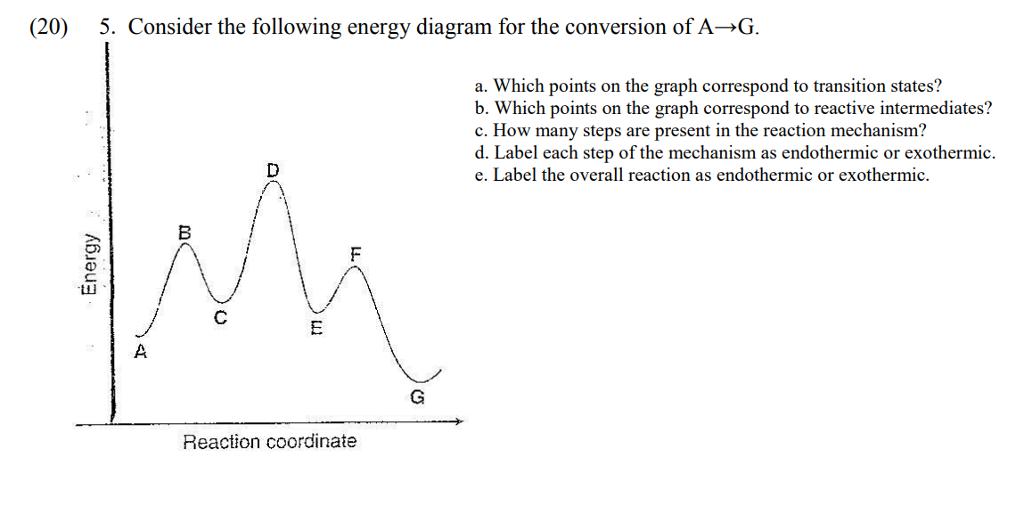






0 Response to "39 consider the energy diagram for the reaction a(g) b(g)"
Post a Comment