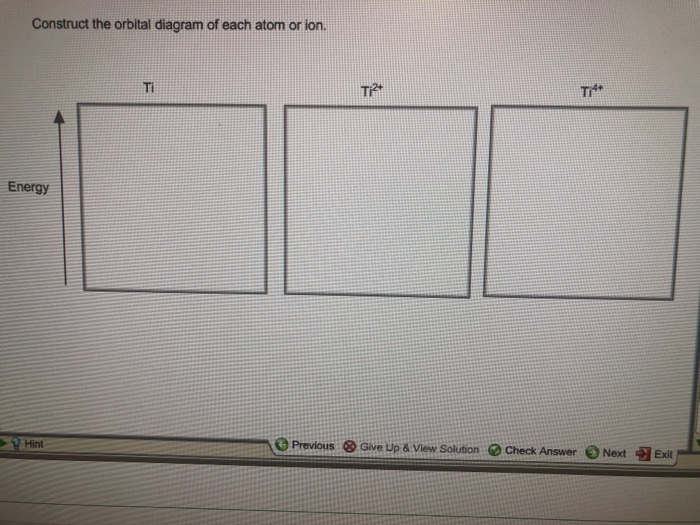
39 construct the orbital diagram of each atom or ion.
Using an orbital filling diagram, predict the order in which electrons will leave their orbitals in order to achieve those valences (i.e. the first ___ electron will come. asked by Haley on May 15, ; Chemistry. Use an orbital interaction diagram to . write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. a. Problem Details. Construct the orbital diagram of each atom or ion. Q. Write the corresponding electron configuration for the following pictorial representation. Give the full electron configuration. Name the element, ass... Q. Create the atomic orbital diagram for nitrogen.
The electron configuration of an atom indicates the number of valence ... configuration for phosphorus and draw the orbital diagram.

Construct the orbital diagram of each atom or ion.
a) Draw the Lewis dot structure for the cyaphide ion and give the formal charges on each atom. Are the formal charges consistent with electronegativity? b) Draw a molecular orbital energy diagram ... For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows must point in opposite directions (representing paired spins). The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund's Rule: Given sev...
Construct the orbital diagram of each atom or ion.. Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (10 ratings) Transcribed image text: Construct the orbital diagram of each atom or ion. What is the electron configuration for titanium? The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. The arrows. Energy levels: 2, 8, 6 Orbitals: 1s2 2s2 2p6 3s2 3p4 If you need to fill in the little boxes, here's one for you. Each arrow represents one electron. Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Practice! Draw an orbital diagram for beryllium (Z=4). 1s. Guidelines for drawing orbital diagrams.
c. The concentration of electrons in the π* orbital is more on the O, so combination with the positive proton at that end is more likely. In fact, H+ bonds to the oxygen atom, at an angle of 97°, as if the bonding were through a p orbital on O. 5.11 The molecular orbital description of KrF+ would predict that this ion, which has the same number 9.40 Draw sketches illustrating the overlap between the fol- lowing orbitals on two atoms: (a) the 2s orbital on each, CQ (b) the 2pz orbital on each (assume that the atoms are on the z-axis), (c) the 2s orbital on one and the 2pz orbital on the other. 9.41 Consider the bonding in an MgH2 molecule. (a) Draw a In the process, each lithium atom [blank] electron(s) and each nitrogen atom [blank] electron(s). three. 67.) Total number of covalent bonds in N2 molecule is [blank] ... Draw the molecular orbital diagram for C2. The number of electrons in the σ2p molecular orbital is ____. ... What diatomic molecule and/or ion(s) would have the molecular ... Figure 7.2.1 Identify Orbital Energies in Single-Electron Species Orbital energies (n = 1 to n = 4) in a single‐electron species For a single-electron species such as a hydrogen atom, the energy of the atomic orbitals depends only on the value of n. For example, a 2p orbital in a hydrogen atom has the same energy as a 2s orbital.
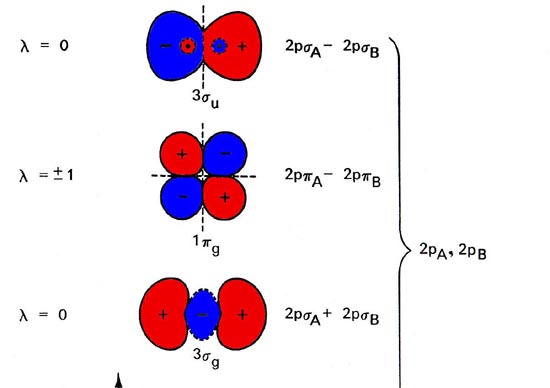
chemical bonding - chemical bonding - Molecular orbitals of H2 and He2: The procedure can be introduced by considering the H2 molecule. Its molecular orbitals are constructed from the valence-shell orbitals of each hydrogen atom, which are the 1s orbitals of the atoms. Two superpositions of these two orbitals can be formed, one by summing the orbitals and the other by taking their difference. Question: Construct the orbital diagram of each atom or ion.TiTi2+. This problem has been solved! See the answer ... Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the ...
Chem 302. The Molecular Orbitals of Ammonia. Determing the electronic structure of ammonia will introduce the new ideas of degenerate orbitals and degenerate axes. It is important to understand these concepts because of the large number of molecules that have point groups such as C 3v or D 3h. To determine the MO's of ammonia.
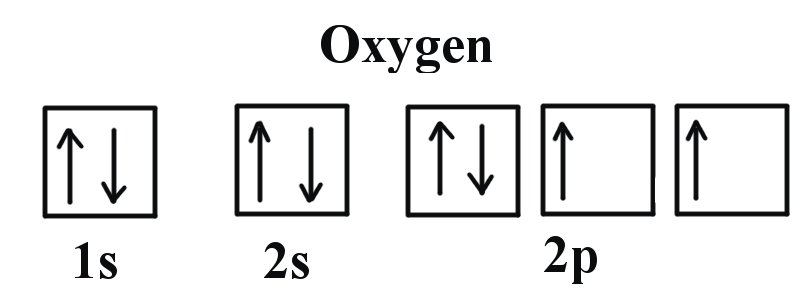
Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. The eighth element in the periodic table is oxygen.
solved construct the orbital diagram each atom ion answer to construct the orbital diagram of each atom or ion ti ti2 ti4. File H2O MO Diagramg. orbital diagram of titanium ti electron configuration this video shows how to draw the orbital diagram of orbital diagram of titanium ti electron configuration and noble gas ti2. My electron box plot.
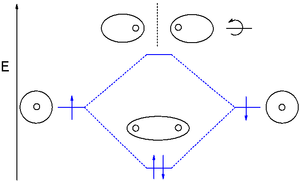
Now we have two of the same atomic orbital diagrams laid out: Then, for the molecular orbital diagram, we examine how these atomic orbitals interact with each other in a linear combination of atomic orbitals (LCAO). Here's how this goes (of course, the #ns# are compatible with the #ns#). Taking the internuclear axis as the #z#-axis, we have:

Show The Orbital Diagrams For The Following Chromium Atom And Chromium Iii Ion Include Only Occupied Orbital S A Cr B Cr 3 Study Com
Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the. Watch the video solution for the question: Draw the orbital diagram for ion Co 2+.. . can be accommodated in the metal d orbitals. • d0 ions •d7 ions - Fe1+, Ru1+, Co2 ...
Generalized energy-level diagram for atomic orbitals in an atom with two or ... construct the ground-state electron configuration and orbital diagram for a ...
FREE Answer to Orbital Diagrams Construct the orbital diagram of each atom or ion. What is the electron configuration...
Chemistry questions and answers. Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxes to add electrons. Question: Construct the molecular orbital diagram for He2 and then identify the bond order.

Solved Molecular Orbital Diagram Of Molecule Is Given Below 111 1k1 1 11 1 1 1 List All Bonding Molecular Orbitals List All Antibonding Molecular Orbitals Identify Degenerate Molecular Orbitals How
Construct the orbital Diagram Of the F- Ion. construct construct the orbital diagram the f- ion chem 120a november 8 2005 fall 2004 8 00 - 9 20 am exam ii name prepare a molecular orbital energy level diagram for no construct a solved construct the orbital diagram the f ion a ne answer to construct the orbital diagram of the f ion a neutral fluorine atom has 9 electrons how many ...
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
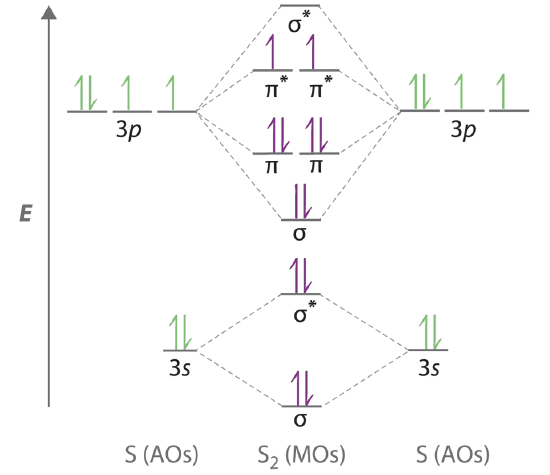
Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11. Each oxygen atom contributes six electrons, so the diagram appears as shown in ...
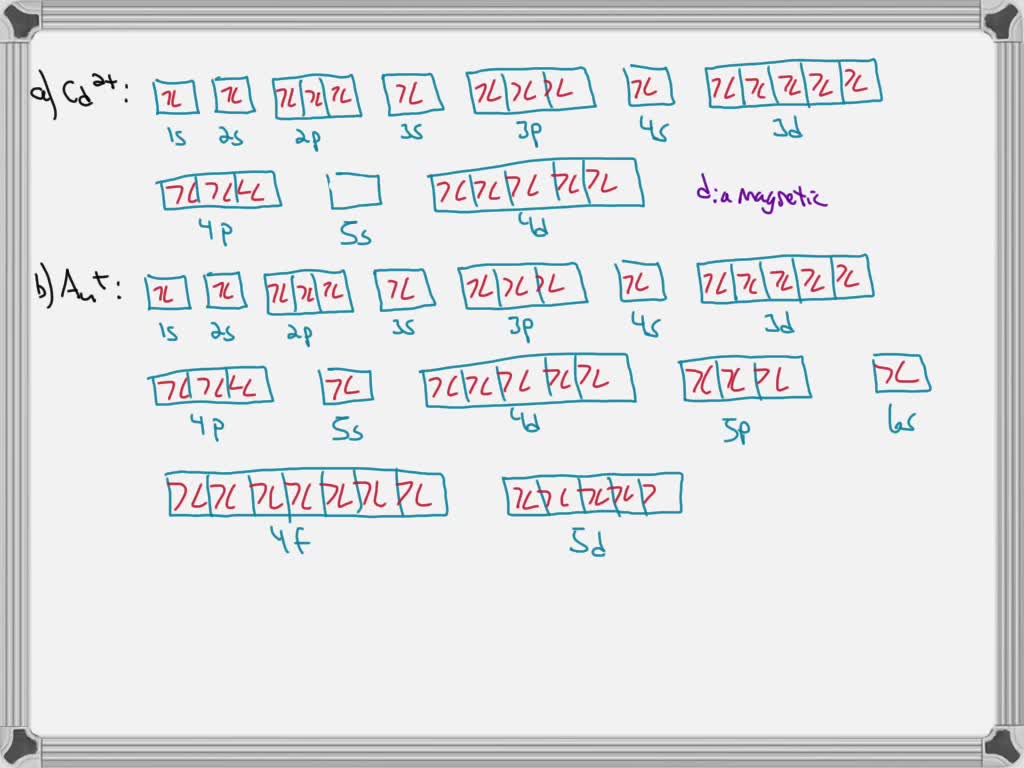
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A Cd2 B Au C Mo3 D Zr2
construct the orbital diagram of each atom or ion. ultramarinetrout400. What is the orbital diagram of each atom or ion? Ti, Ti 2+, Ti 4+ ... Get the detailed answer: Match each diagram to the atom or ion it represents? 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → ...
Orbital Diagram. Orbital diagrams are ways to assign electrons in an atom or ion. Each atomic orbital is represented by a line or a box and electrons in the ...
In a neutral atom, the number of protons is equal to the number of electrons. So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is 1s22s22p63s23p64s1. The noble gas notation is [Ar]4s1. The following orbital diagram shows the increase in energy from one energy sublevel to the next ...
Orbital Diagram Of Ti2+. Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+. Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . When filling degenerate orbitals, electrons fill them singly first, with parallel spins is ...
Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals 1. Begin with the Lewis structure. 2. Decide how many orbitals each atom needs to make its sigma bonds and to hold its non-bonding electrons. Draw the atomic and hybrid orbitals on on side of the page. 3. For each sigma bond, take a hybrid (or atomic) orbital from ...
Draw the orbital electron filling diagram, using the shortcut of (noble gas) to represent core electrons and up/down arrows to indicate all other electrons, for the atom with atomic number 82 ...
Answer (1 of 4): Nickel is atomic number 28; therefore, it has 28 electrons in its orbitals. The filling rules are as follows: 1. Aufbau Principle: Lowest energy levels fill first. 2. Pauli Exclusion Principle: Only 2 electrons per orbital, they must have opposite spin. 3. Hund's Rule: Given sev...
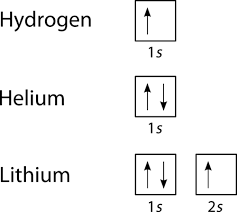
For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows must point in opposite directions (representing paired spins). The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom.
a) Draw the Lewis dot structure for the cyaphide ion and give the formal charges on each atom. Are the formal charges consistent with electronegativity? b) Draw a molecular orbital energy diagram ...

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
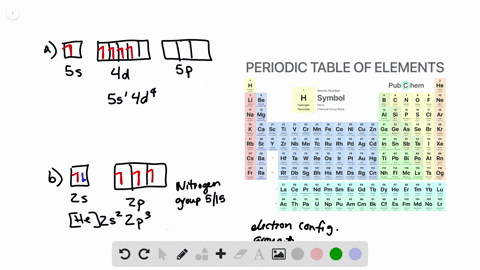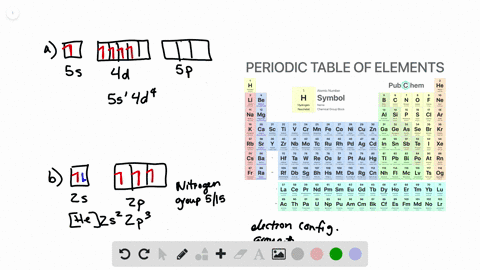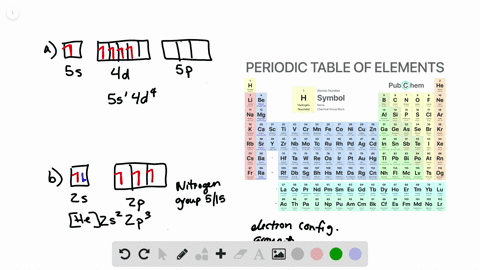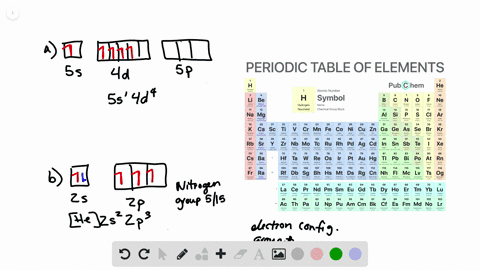
Solved Write Orbital Diagrams For The Valence Electrons And Indicate The Number Of Unpaired Electrons For Each Element A Ne B I C Sr D Ge



















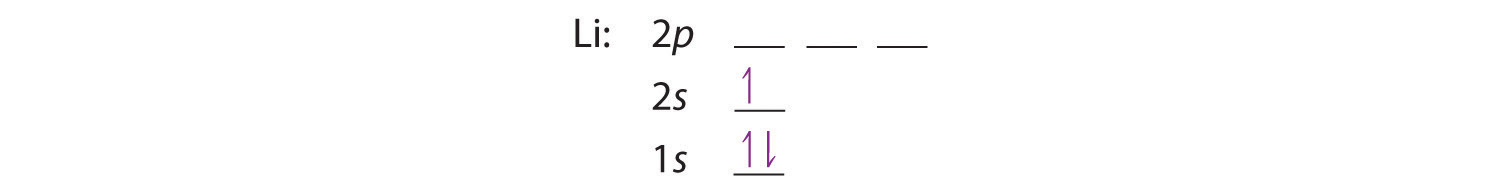

0 Response to "39 construct the orbital diagram of each atom or ion."
Post a Comment