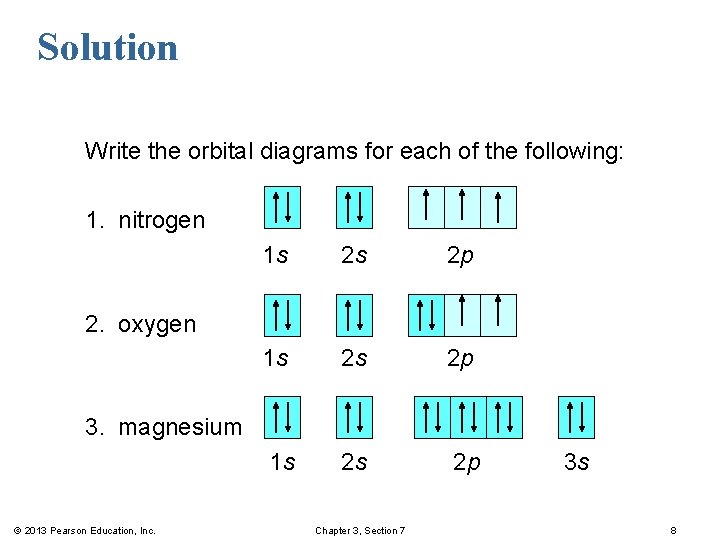
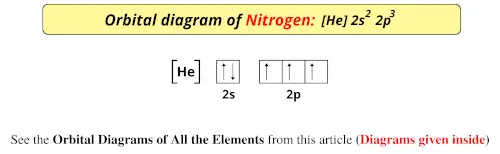
36 orbital diagram for nitrogen
The orbital can be found in every energy level except the first two. The correct electron configuration for calcium is 1) 1s22s22p63s23p63d2 2) 1s 22s 2p63s23p64s2 3) 1s 22s 2p63s23p8 Learning Check Nitrogen appears as a colorless odorless gas. For example, the Lewis electron dot diagram for calcium is simply ...
Figure 1. π-molecular orbital diagrams for (a) benzene, (b) fluorobenzene, (c) m-difluorobenzene, (d) 1,3,5-trifluorobenzene, (e) 1,2,3,5-tetrafluorobenzene, (f) pentafluorobenzene, and (g) hexafluorobenzene. Conventional aromatic orbitals are shown in black while additional π-orbitals formed by the conjugation of substituents to the ring are ...
Naim was happy, Naim felt safe, he crawled through a duct between decks and found the issue Mick had chirped at him, with his deft claws he unhooked a data cable unwound the fixing piece and carefully stripped back the clear wires, Naim did not know why these wires were clear, some had liquid in them others seemed to be filled with light. It didn't matter to him, as long as the Goddess was happy so was Naim, as he worked, fitting each strand into a connector. It amused him that he could recogni...
Orbital diagram for nitrogen
Electron configuration of oxygen diagram. 1s²2s²2p⁴ the orbital diagram has five boxes with two arrows in the first three and single arrows in the last two.N atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.
So I need someone to check some of these, so I might crosspost this to other subreddits, if you know any, please do. Or if you are an expert yourself, please correct me if there's any mistakes. But I did watch Dr. Stone in an *Anime Streaming website*, I posted some interesting comments in the discussions of Dr. Stone Episodes. I will post them in a chronological order with the matching episodes. Although, I think it's a bad idea to post this in a whole one post. Because no one gonna read it t...
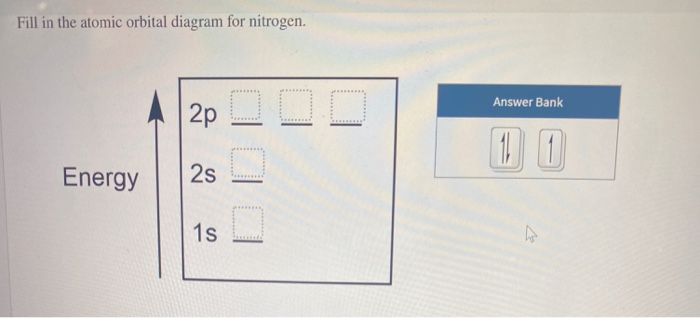
Write the orbital notation for nitrogen. 5 Bellwork - 1/27 The electron configuration of boron is 1s2 2s2 2p1. What is the atomic number for boron? ... Write the orbital diagram and electron configuration for any 1st or 2nd period element. Write the noble gas configuration for any 3rd or 4th period elements!
Orbital diagram for nitrogen.
At the moment, I can’t think of anything else to say on this mars spacecraft concept, so let’s move to landers. There would be a small multistage lander to bring the crew from high mars orbit to low mars orbit to the planetary surface back up to low and then high orbit, and a larger habitat lander to deposit the necessary living volume and consumables for a potentially several hundred day surface stay ahead of time. Of these, the crew lander is going to be much easier to depict with the tools av...
An OpenStax CNX book
##*“Go ahead and run you swine, but first I’d like to teach you something about aesthetics. This is what a real world class villain looks like, you son of a bitch.”* #[Accelerator](https://i.imgur.com/ihGwsMS.png) Accelerator is the #1 Rank 5 Esper in Academy City, and secondary protagonist of the series. Once seeking nothing but the power to become a Level 6, he learned the value of protecting someone after meeting Last Order. Since then, he went on a journey from a villain, to a villain hun...
40 strip diagram anchor chart; 37 orbital diagram for barium; 37 4 string day night shade diagram; 42 create the atomic orbital diagram for nitrogen. 39 1955 chevy ignition switch wiring diagram; 41 john deere 325 parts diagram; 41 choose the correct orbital diagram for vanadium. 38 chevy s10 stereo wiring diagram; 42 john deere d110 belt diagram
Say you want to buy my house, but you're out of money. What can you do? There are some obvious things, like getting a job or taking out a loan, but those things bore you, so here's an interesting solution: you do something extraordinary that convinces me to trust you more than my wife. Then you sign a piece of paper saying: "I owe you one." Assuming what you did to make me trust you was public enough, I might not even need to cash in your promise. Instead, I can go to someone else and han...
The diagram showing orbital overlapping in the ammonia NH3 molecule The orbitals of NH3 participating in the bond formation to undergo sp3 hybridization Molecular orbital diagram of ammonia ... and there is one pair of nonbonding electrons on the nitrogen atom. The molecular orbital diagram is a diagrammatic representation of how chemical ...
My question is asking to describe the bonding in the NO2- ion using valnece bond theory versus Molecular Orbital theory. I know that the ion is bent and that there are 2 sigma bonds and 1 pi bond and that the nitrogen is sp2 hybridized. But the latter part of my question asks how MO theory describes the pi bond in the species. This is a general chem course and I frankly have no clue how to go about drawing a MO diagram for a non-diatomic molecule, so I’m guessing there’s something else I have...
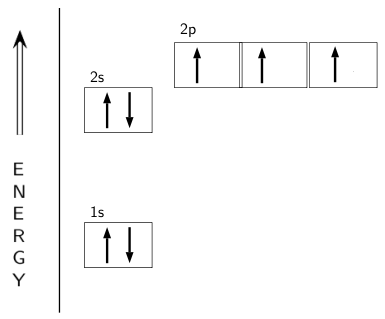
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms.
A guide for alien Species concerning Humans by a Human ​ Written in Union Standard Machine Communication Language (USMCL) by Henry Katou Vizex Machine Translator recommended for reading ​ Section 1: Common misconceptions regarding Hummans ​ I have met individuals of a grand total of 3 non-Human Species and have already discovered a plethora of astonishing misconceptions about my Species. I am a humble molecular network engineer, but I would like to offer t...
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms.
>I'm keeping the original post in tact, but be sure to check the edits! Some changes are being made... > >Also! Thank you so much for the silver et al, and for all the great advise! A lot of people are explaining that we had plastics in antiquity if you consider anything malleable ("plastic") or anything with polymers to be plastic. Thanks for the learning opportunity! But to be clear, I intended to mean oil-based plastic, like (in this case) polyethylene: what our plastic bags and ...
The alkali steel lithium reacts v nitrogen(N2) in ~ high temperatures to type nitride compounds. 6Li + N2 → 2Li3N. The superior properties the lithium atoms. ... Lithium electron configuration and also orbital diagram are the key topics that this article. Also, duration and group determination, valency and valence electrons, assorted ...
37) The orbital diagram for fluorine shows 1 unpaired electron in a p orbital. 38) The correct electron configuration for magnesium is: 1s 2 2s 2 2p 6 3s 3 . 39) The element manganese (symbol = Mn) has five valence electrons.
For example, [Ne] represents the 1 s 2 2 s 2 2 p 6 electron configuration of neon ( Z = 10), so the electron configuration of sodium, with Z = 11, which is 1 s 2 2 s 2 2 p 6 3 s 1, is written as [Ne]3 s 1: Neon. Z = 10. 1 s 2 2 s 2 2 p 6. Sodium. Z = 11. 1 s 2 2 s 2 2 p 6 3 s 1 = [Ne] 3 s 1.
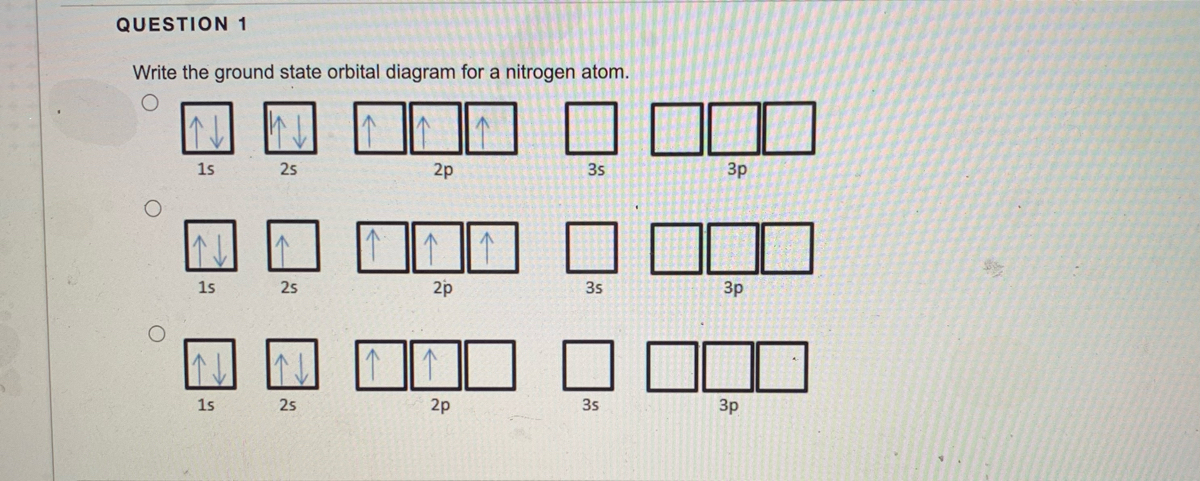
(10) Following orbital diagram shows the electron configuration of nitrogen atom. Which rule does not support this? (AS1) N (Z = 7). Ans: -The orbital diagram of the N doesn't support the hunds principal where it clear that electron fill in the orbital equal energy. But in this case the third orbital is empty which violet the principal.
Hydrogen fluorine nitrogen hydrogen fluoride carbon monoxide methane ammonia ethylene acetylene allene formaldehyde benzene. ... The molecular orbital diagram for carbon monoxide figure pageindex1 is constructed similarly to how you would construct dicarbon or dioxygen except that the oxygen orbitals have a lower potential energy than analogous ...
Orbital Diagram: The subatomic particle that occupies most of the volume of an atom is known as the electron. The region of space wherein electrons ... That is, magnesium is a cation element. Magnesium donates the electron of the last shell to form bonds and turns into magnesium ions. Mg - 2e - → Mg +2.
This tells us that each subshell has double the electrons per orbital. The s subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3 orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold up to 10 electrons, and the f subshell has 7 orbitals with 14 electrons. Example 1: Hydrogen and Helium.
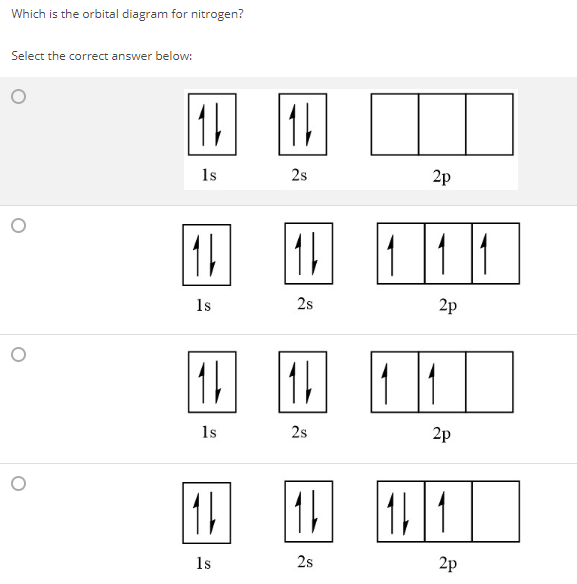
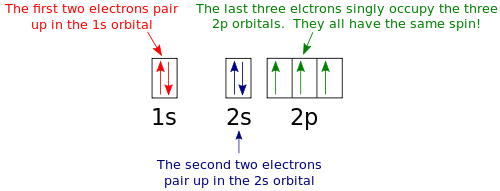
Draw an orbital diagram for nitrogen, Z = 7. What is the electron configuration of this atom? Answer. When we get to nitrogen (Z = 7, with seven electrons), Hund's rule tells us that the lowest-energy arrangement is. with three unpaired electrons. The electron configuration of nitrogen is thus 1s 2 2s 2 2p 3.
The molecular orbital diagrams of , and are drawn in the attached image. ... For lighter elements, such as Boron, carbon, nitrogen, the π2py and π2pzare less in energy than the σ2px and the σ*2px is lesser in energy than the π*2py and π*2pz orbitals. For these atoms the order is;
NCl3 is the chemical formula for Nitrogen trichloride. Also, called trichloramine it is a halogen nitride that is yellow and oily with a pungent smell. It is ... For nitrogen atom, one 2s orbital and three 2p orbital mixes and intermixes produce four new hybrid orbitals of similar energy levels.
Diatomic nitrogen is more complex than hydrogen since multiple molecular orbitals are occupied. Four molecular orbitals are occupied (the two \(1\pi_u\) orbitals are both occupied). ... Important aspects of molecular orbital diagram in Figure 10.4.5 : The H 1s energy lies well above the Cl 2s and 2p atomic orbitals;
Crystal structure of Nitrogen. HEX (Hexagonal) Melting point of Nitrogen (N 2) 63.23 K or -209.86 °C or -345.75 °F. Boiling point of Nitrogen (N 2) 77.35 K or -195.79 °C or -320.43 °F. Density of Nitrogen. 0.808 g/cm 3 (when liquid at barometric pressure) Main isotope of Nitrogen.
Some of them are S-orbital, P-orbital, and D-orbitals. However, it is important to have a knowledge of which element will fall in which orbital, and these things you can know by using our table orbitals. How is The Periodic Table Organized. Now, most of the students might always wonder why the structure of the table is designed this way.
Nitrogen belongs to group 15 and has 5 valence electrons. Hydrogen belongs to group 1 and has 1 valence electron. Total number of valence electrons in N2H2 = 5*2 + 1*2 = 12. ... Molecular Orbital (MO) Diagram. In VBT, also known as Valence Bond Theory, we consider the fact that atomic orbitals ( AOs ) from the same individual atom can come ...
The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital. The bonding MO is occupied by two electrons of opposite spin, the result being a covalent bond.
To fill the diagram, first, we fill each side of the diagram with the electrons according to nitrogen's electron configuration - [He]2s 2 2p 3. Next, we fill the middle section with the molecular orbital's electron configuration using Hund's Rules , just as we do with atomic orbitals.
Draw the molecular orbital diagram for B 2. The number of unpaired electrons in the B 2 molecule is _____. (a) zero (b) 1 (c) 2 (d) 3 (e) 4 8. Which one of the following statements is false? (a) Valence bond theory and molecular orbital theory can be described as two different views of the same thing.
Nitrogen is the chemical element with the symbol N and atomic number 7. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772. ... Molecular orbital diagram of dinitrogen molecule, N 2. There are five bonding orbitals and two antibonding orbitals (marked with an asterisk; orbitals involving the inner 1s electrons ...
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
09/06/2017 · molecular orbital diagram for nitrogen gas (n2)use aufbau and hund to fill with 10 valence electronsyou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).bond or. For the second period elements the 2s and 2p orbitals are important for mo considerations. 31/03/2021 · 12+ n2 molecular orbital diagram. 07/08/2021 · do you know !!
There are 5 valence electrons in Nitrogen Electron Configuration and it lies at the top of group 15 in the periodic table. Apart from that one ...15 Feb 2021 · Uploaded by Wayne Breslyn
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.
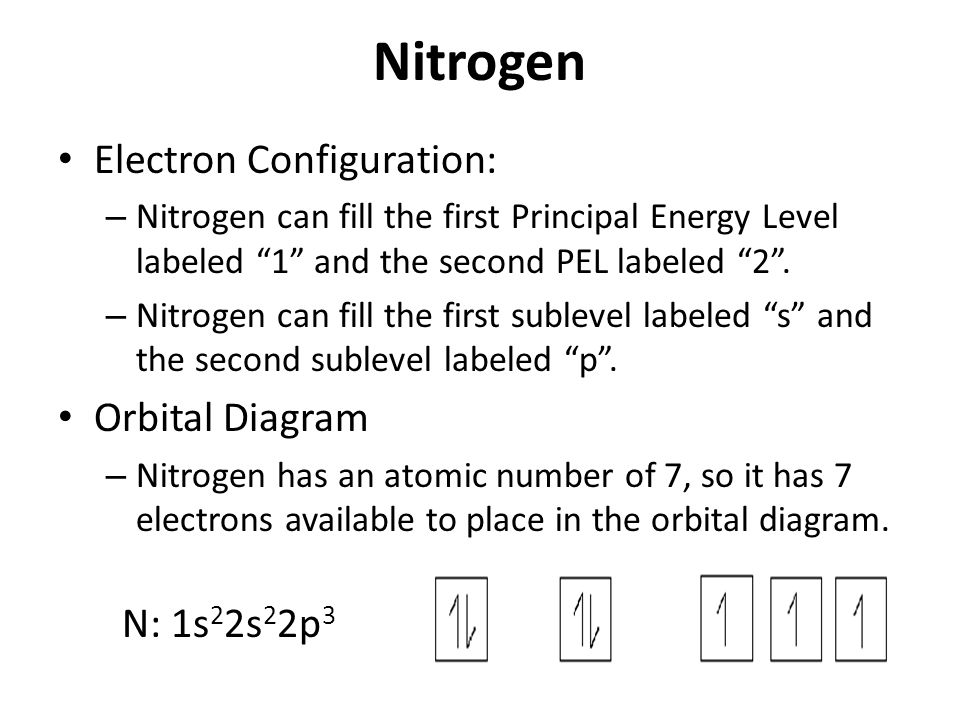
Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p.Show transcribed image text Show the orbital-filling diagram for N. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital.
In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...24 Oct 2016 · Uploaded by Wayne Breslyn
Mar 04, 2021 · The lithium 1s orbital is the lowest-energy orbital on the diagram. Because this orbital is so small and retains its electrons so tightly, it does not contribute to bonding; we need consider only the 2 s orbital of lithium which combines with the 1 s orbital of hydrogen to form the usual pair of sigma bonding and antibonding orbitals.
What is the atomic orbital diagram for nitrogen? The remaining three electrons will go in the 2p orbital. Therefore the N electron configuration will be 1s22s22p3. The configuration notation for Nitrogen (N) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the Nitrogen atom.
21 Jan 2021 — When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.


















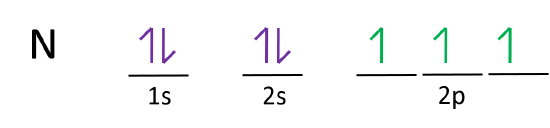


0 Response to "36 orbital diagram for nitrogen"
Post a Comment