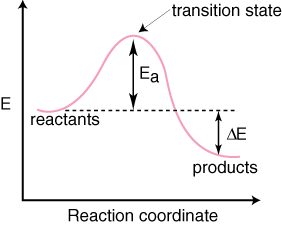
38 exothermic reaction coordinate diagram
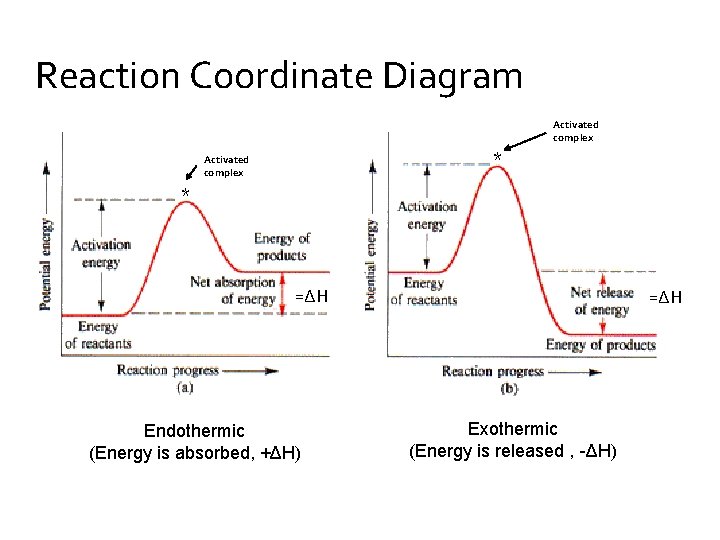
Exergonic and endergonic reactions are characterized by changes in Gibbs energy. In the equilibrium state of an exergonic reaction, the Gibbs energy of the products is lower than that of the reactants. Meanwhile, the equilibrium state of an endergonic reaction in, the Gibbs energy of the products is higher than that of the reactants.
D G and Direction of Reactions. To see how D G values can be used to predict how reactions will go, consider the four cases you encountered in the Entropy Module: . 1. D H 0, D S > 0: . This is an exothermic reaction with an increase in entropy. Thus D S univ > 0 and D G 0. The Second Law of Thermodynamics says that such a reaction is product-favored, so a D G less than zero also means a ...
Exothermic Reaction Coordinate Diagram. More Details . Scosche Fdk106 Wiring Diagram. More Details . 2008 Aliner Wiring Diagram. More Details . Western Plow Unimount Wiring Diagram. More Details . John Deere X380 Wiring Diagram. More Details . 1989 Peterbilt 379 Wiring Diagram. More Details .
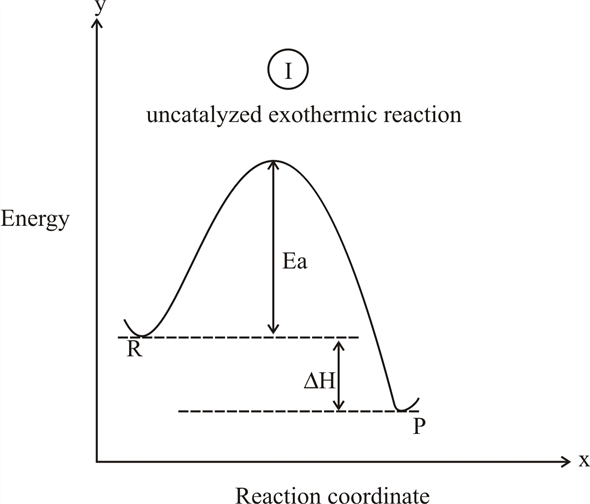
Exothermic reaction coordinate diagram
Free Chemical Reactions calculator - Calculate chemical reactions step-by-step
question 100 deals with being able to draw a reaction. Coordinate diagram simply from information that is provided. It asks you to draw on eggs. A thermic reaction coordinate diagram that occurs in a single step. If it's a single step, then there's one hump. If it's an extra thermic reaction than were lower energy in the products than where we started, the activation energy corresponds to the ...
Endothermic Reaction Examples. When ammonium chloride (NH 4 Cl) is dissolved in water, an endothermic reaction takes place. The salt dissociates into ammonium (NH 4+) and chloride (Cl -) ions. The chemical equation can be written as follows: NH4Cl (s) + H2O (l) NH4Cl (aq) - Heat. Ammonium nitrate (NH 4 NO 3 ), an important component in ...
Exothermic reaction coordinate diagram.
Asoccer player practices kicking the ball into the goal from half way down the soccer field. the time it takes for the ball to get to the goal from the time it is kicked averages about three seconds. the soccer field is 90 meters long the player wants to know the average velocity of the ball. is there enough information to calculate this ?
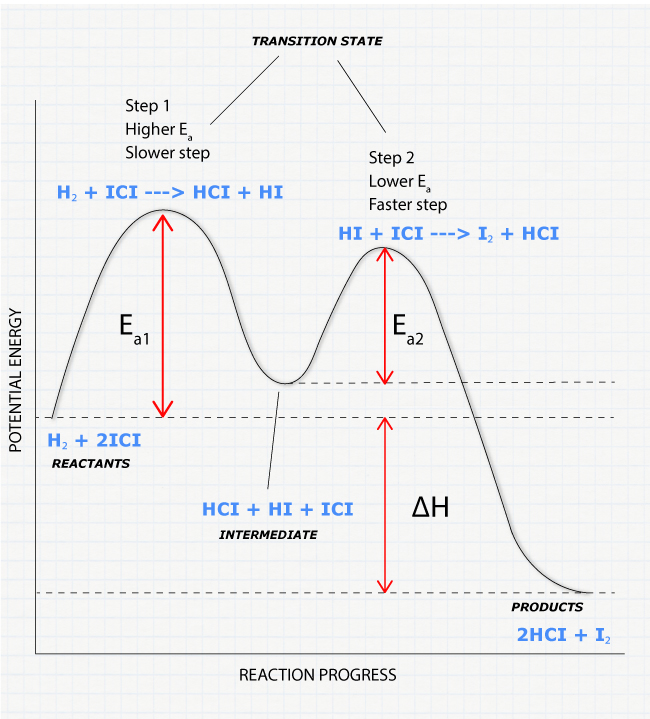
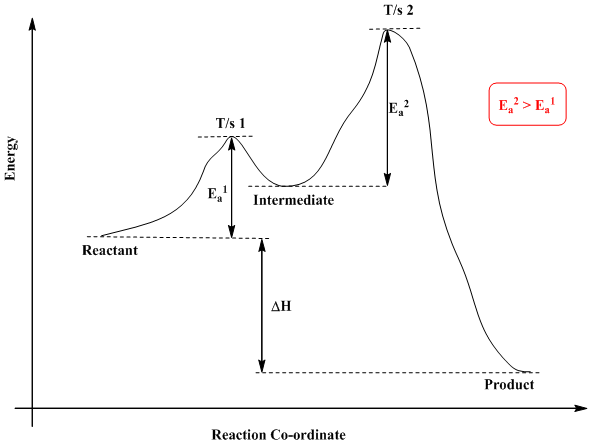
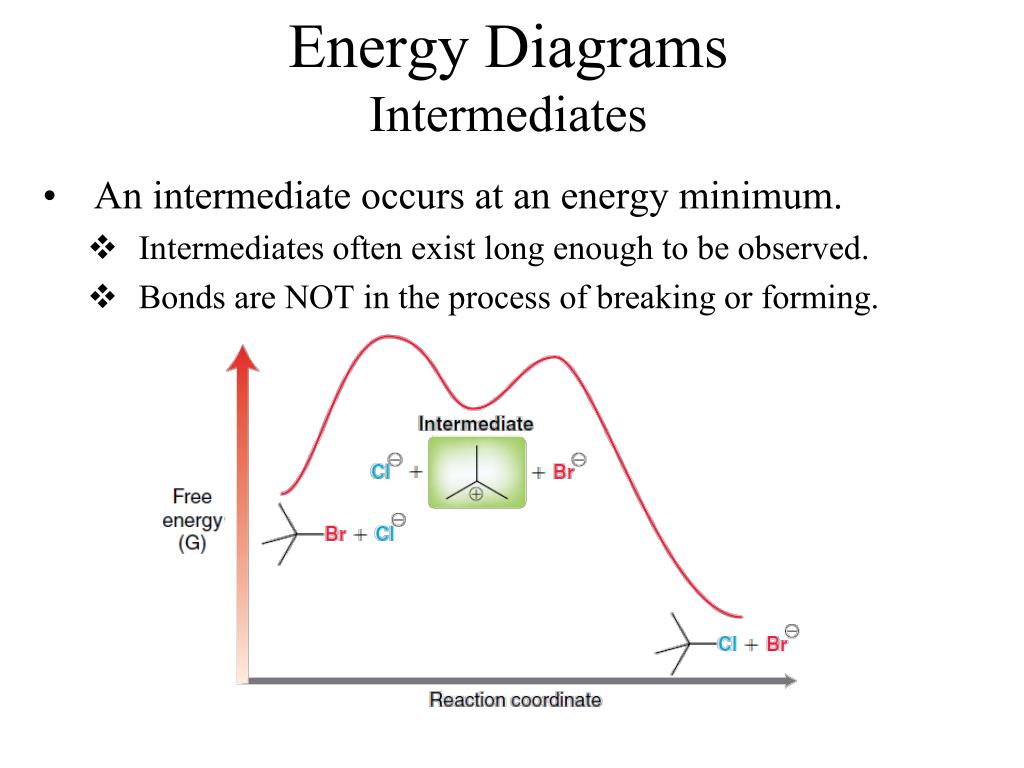
A Two-Step Reaction Mechanism. The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall. Step 1 has the higher transition energy state, thus it ...
Energy level diagram for an exothermic reaction is shown below. Endothermic reactions take in energy and the temperature of the surroundings decreases. Energy is being put in to break bonds in the reactants. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled.
In this video, I go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. I'll ...
Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
3 | P a g e Exothermic and endothermic reactions When a chemical reaction happens, there can be a net transfer of energy from the reactants into
Draw a reaction coordinate for the following catalyzed two-step exothermic reaction with the second step being the rate determining step:A -- B
Solutions for Chapter 14 Problem 96SCQ: Draw a reaction coordinate diagram for an exothermic reaction that occurs in a single step. Identify the activation energy and the net energy change for the reaction on this diagram. Draw a second diagram that represents the same reaction in the presence of a catalyst, assuming a single step reaction is involved here also.
Learn about exothermic and endothermic reactions and the transfer of energy with GCSE Bitesize Chemistry (AQA).
Learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction.
Bond energy is the amount of energy absorbed to break the bonds or released energy during the formation of bonds in one mole of the substance . The breaking of bonds is an endothermic process and needs to absorb an amount of energy from the surrounding , So , its ΔH has a positive sign .
An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings.". Based on the above definition, let's pick a few examples from our daily lives and categorize them as endothermic or exothermic.
Arrhenius Law and Temperature Dependence. The rate of a reaction depends on the height of the barrier (the activation energy) because only a small fraction of the number of molecules at a given temperature have sufficient energy to get over the barrier.
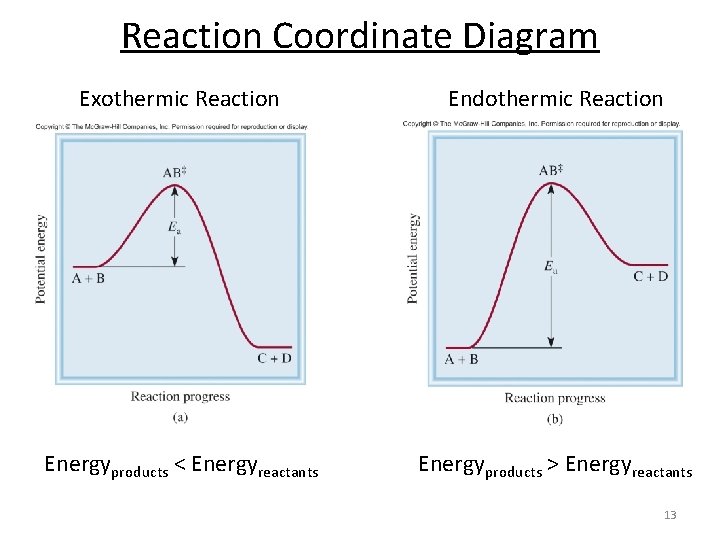
Exothermic Reaction. Endothermic Reaction. Reaction Coordinate Diagram. Energyproducts > Energyreactants. Energyproducts . Energyreactants.. The " reaction coordinate " plotted along the abscissa represents the diagrams can describe both exothermic and endothermic reactions.
This diagram of a chemical reaction shows that the reaction is___. Endothermic Exothermic Reversible At equilibrium 1 2 3 4 5.
Enthalpy change is the difference between the energy contents of the products and reactants when a reaction occurs. There are two types of enthalpy changes exothermic (negative enthalpy change) and endothermic (positive enthalpy change). The unit of enthalpy change is Kilojoule per mole (KJ mol-1).
The energy diagram above details endothermic reactions, where the total beaker ... If the reaction is exothermic, heat is released during the course of the ...
Always to the left of the arrow in a chemical equation. In exothermic reactions, there is more energy in the reactants than in the products. In endothermic reactions, there is less energy in the reactants than in the products.
19 Dec 2007 — S-1 RF SYSTEM SCHEDULE & CABLING SCHEMATIC DIAGRAM ... EXOTHERMIC WELDS SHALL BE MADE IN ACCORDANCE WITH ERICO PRODUCTS BULLETIN A-AT.
This problem has been solved! See the answer. Draw a reaction coordinate diagram for 3 step exothermic reaction, that occurs with the formation of the first intermediate being the rate limiting step. Label the diagram by indicating the reactants, products, all intermediates and all transition states. Show transcribed image text.
11 Dec 2020 — 8. JOISTS SHALL BE PLACED DIRECTLY OVER BEARING STUDS AND JOIST WEB STIFFENERS SHALL BE PROVIDED AT ALL REACTION POINTS AND AS. OTHERWISE SHOWN ...30 pages
Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. The reaction coordinate is a parametric curve that follows the pathway of a reaction and indicates the progress of a reaction.
by O COUNTY · 2016 — reaction. Reconnect sensor. Make a reading with a calibrated test instrument within 6 inches of the site sensor.389 pages
Energy diagrams for these processes will often plot the enthalpy (H) instead of Free Energy for simplicity. The standard Gibbs Free Energy change for a reaction can be related to the reaction's equilibrium constant (\ (K_ {eq}\_) by a simple equation: (6.6.2) Δ G ˚ = − R T ln. . K e q.
An applicant's safety review document must contain a block diagram that identifies all flight ... (2) A flight safety official's decision and reaction time, ...
In other words, the products are less. A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change there are many ENDOTHERMIC reactions such as photosynthesis that occur.! An Exothermic (Exergonic) Reaction! 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O! A reaction with ∆H°0 is endothermic.
by DJ Bender · 1978 · Cited by 32 — Figure 4-1 is a power flow diagram for the reactor with the average equi ... The fusion reaction rate is only weakly dependent on E. in the ...
Exothermic Reaction Coordinate Diagram. reaction coordinate diagrams butaneem uiuc in this example b is at a lower total energy than a this is an exothermic reaction heat is given off and should be favorable from an energy standpoint the energy difference between a and b is e in the diagram however some energy is required for a to convert to b ...
If you have a chemical reaction or a phase change, how might you use the LC sheets to determine whether it was an endothermic or an exothermic reaction? Page 30 ...58 pages
Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction. This is primarily due to the very high energy (low stability) of the oxygen atom that is produced. The oxygen atom produced has a higher energy than ground state oxygen.
21 Sept 2021 — Thus, the mixing processes in the mixtures of 0.25 w 1 0.90 are enthalpy-driven because the exhibited exothermic natures.
Exothermic Reaction Coordinate Diagram. reaction coordinate diagrams butaneem uiuc in this example b is at a lower total energy than a this is an exothermic reaction heat is given off and should be favorable from an energy standpoint the energy difference between a and b is e in the diagram however some energy is required for a to convert to b this energy is called the activation energy or e ...
reaction. C) the average kinetic energy of the reactants. and products ... Given the potential energy diagram for a chemical ... reaction is exothermic.

Which reaction coordinate diagram represents a reaction in which the activation energy, ea, is 50 kj•mol –1 and the δhrxn is –15 kj•mol –1?

The following reaction coordinate diagram, represents, an exothermic reaction, endothermic reaction, neither a nor b, \(\delta h\) is less than zero
























0 Response to "38 exothermic reaction coordinate diagram"
Post a Comment