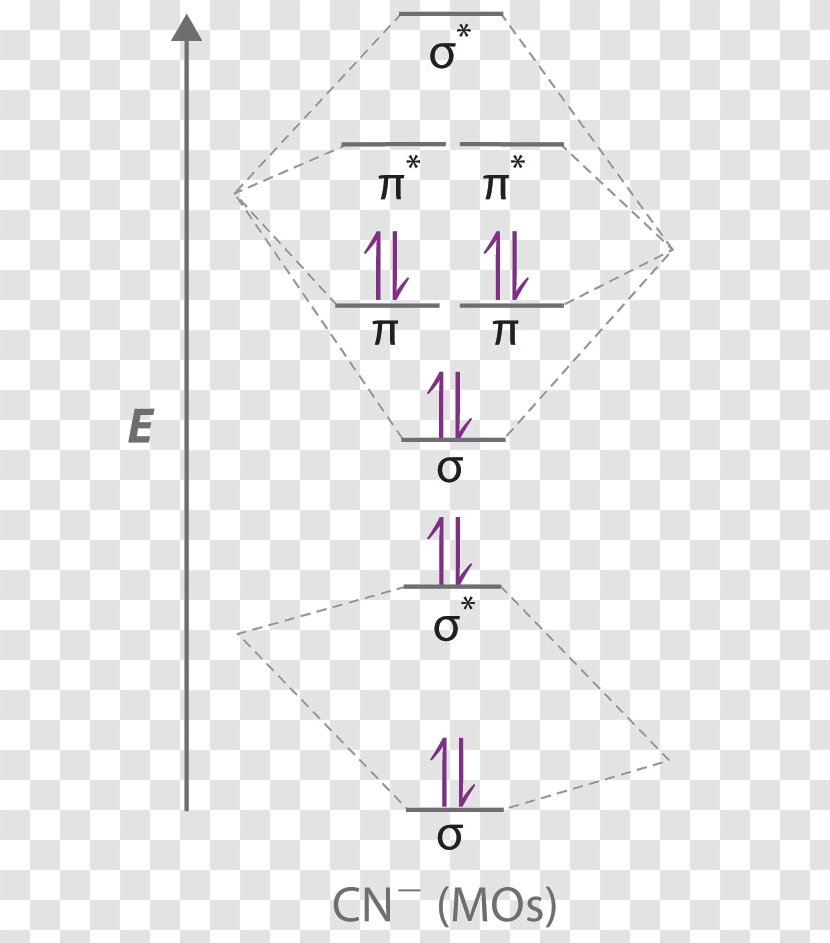
40 cn molecular orbital diagram
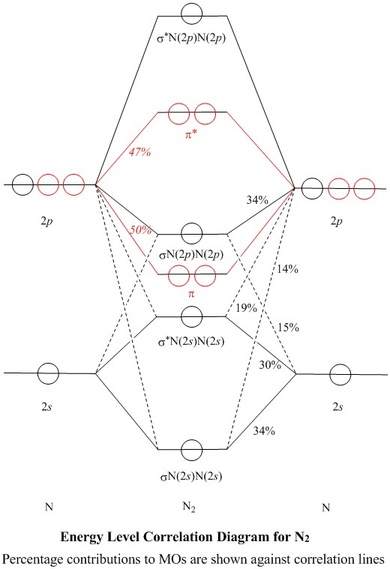
39. The energy of σ2pz molecular orbital is greater than π2px and π2py molecular orbitals in nitrogen molecule. Write the complete sequence of energy levels in the increasing order of energy in the molecule. Compare the relative stability and the magnetic behaviour of the following species : N 2, N 2+, N 2-, N 2 2+ Solution: In the second diagram only sigma bonding is considered and it shows the combination of the metal 3d, 4s and 4p orbitals with OCCUPIED ligand group orbitals (using 1 orbital from each ligand). The result is that that the metal electrons would be fed into t2g and eg* molecular orbitals which is similar to the CFT model except that the eg orbital ...
Aug 10, 2020 · Kohn–Sham molecular orbital analysis revealed that the ... Although NiPc–OMe-H 2 required the least energy to generate *COOH from the free energy diagram, NiPc–CN-H 2 was generated with the ...

Cn molecular orbital diagram
The hybrid orbitals are more prominent outward so that their ability to overlap is stronger than that of normal orbitals. Molecular Formula: A chemical formula is a brief way of expressing the number and type of atoms that make up a particular chemical compound. Walsh diagram OH 2, SH 2, NH 2 8 Bent-, FH2 + NH 2, PH 2, CH 2 7 Bent-, OH2 + CH 2, SiH 2, BH 2 6 Bent-, NH2 + BH 2, AlH 2, CH 2 5 Bent BeH 2, BH 2+ 4 Linear LiH 2, BeH 2+ 3 Linear LiH 2+ 2 Bent No. of Shape valence electrons Molecular species Known shape of some AH 2 molecules Recall: a molecule adopts the structure that best stabilises the HOMO. Molecular orbital Diagram Cn-mo diagram of cn hunt research group right you have been asked to draw the mo diagram for cn a heteronuclear diatomic don t panic take it one step at a time and you will have a plete mo diagram before you know it this is meant to be an interactive exercise so arrange for some pieces of blank paper a pencil a pen and an eraser molecular orbital theory heteronuclear ...
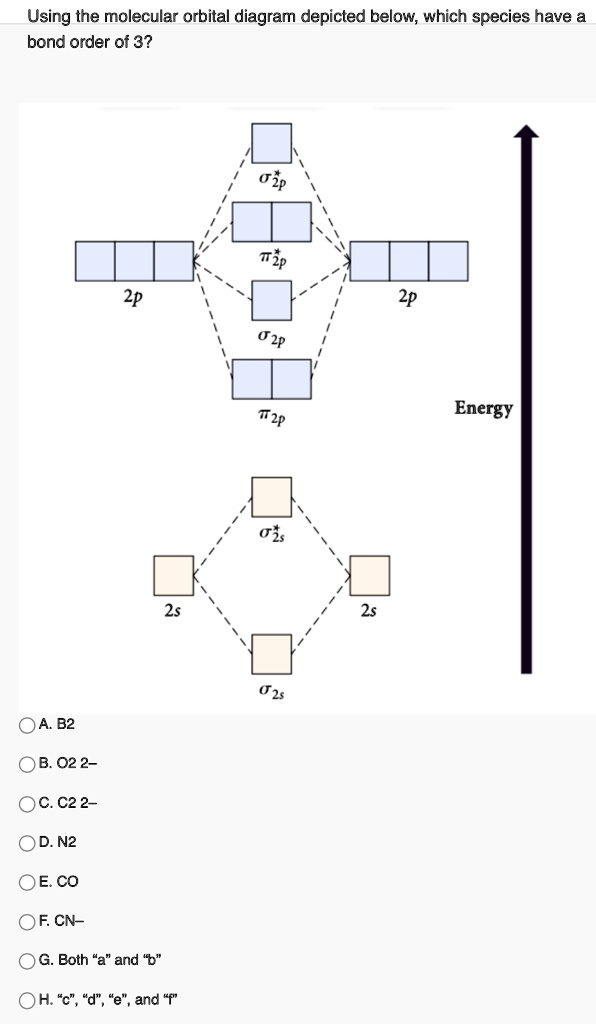
Cn molecular orbital diagram. Problem: Use the molecular orbital diagram to figure out the electronic configuration for CN. Which of the following statements is correct?a) CN is diamagnetic.b) CN− is paramagnetic.c) If an electron is removed to give CN+, the bond order increases.d) The π*2p orbital is the highest energy orbital containing an electron in CNe) If an electron is added to give CN−, the bond length decreases. Home / Structure and Bonding / Atomic Orbitals / Molecular orbitals in Carbon Monoxide. Molecular orbitals in Carbon Monoxide. CONTROLS > Click on the CO molecular orbitals in the energy level diagram to display the shapes of the orbitals. Explore bonding orbitals in other small molecules. When two atomic orbitals combine, two molecular orbitals are formed. One is known as bonding molecular orbital and the other is called an anti-bonding molecular ...1 answer · Top answer: Concepts and reason • The molecular orbital theory explains the bonding in terms of the combination and organization of atomic orbitals of an atom which ... Question: Complete the molecular orbital diagram for CN. Note that the 1s orbitals are not shown. Identify the bond order of CN. O2 01 OOOOO 25- 0 2s Answer Bank The atomic orbitals on the left side of the molecular orbital diagram are those of The atomic orbitals on the right side of the molecular orbital diagram are those of.
Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals. Answer (1 of 5): The total number of electron of CN- ion is ( 6+7+1) = 14 . According to molecular orbital theory, the electronic configuration of CN - ion is as follows, From the above electronic configuration , it has been found that , the number of bonding electron is 10 and the the number of... The molecular orbital diagram of CN molecule is shown in the following figure: Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition. Molecular Orbitals for Heteronuclear Diatomic Molecules (MO Theory) Reviewed by تعرف على علم الكيمياء on 5/20/2019 Rating: 5 Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
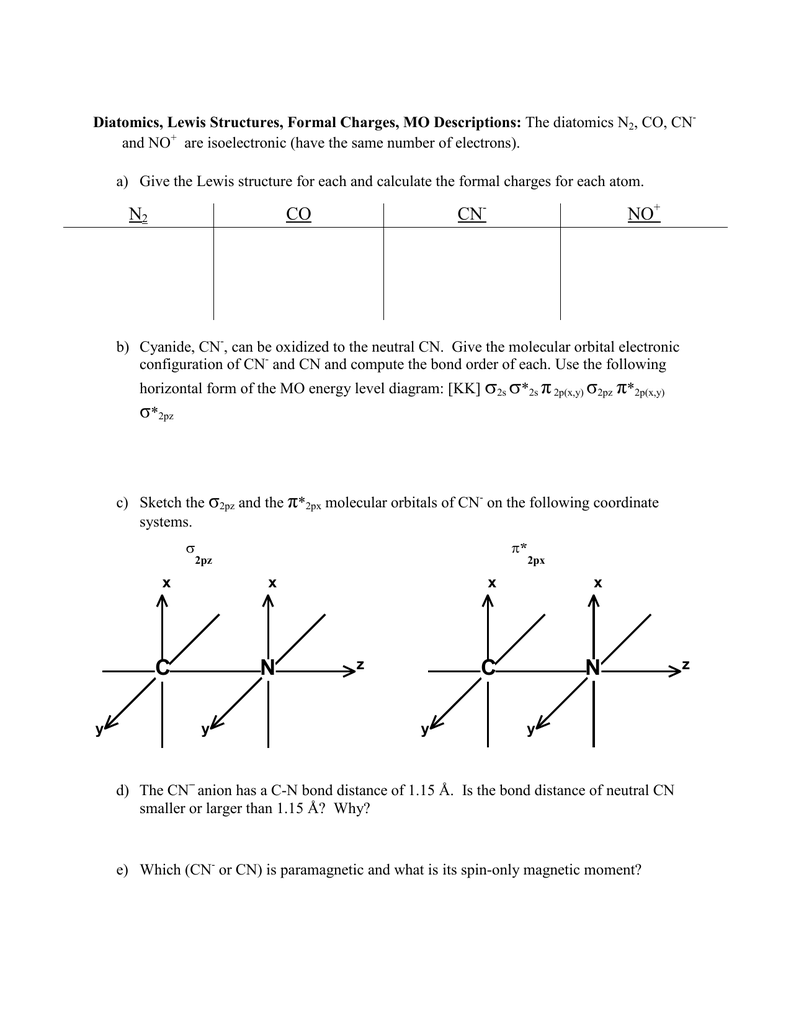
A molecular orbital model for 1,3-butadiene is shown below. Note that the lobes of the four p-orbital components in each pi-orbital are colored differently and carry a plus or minus sign. This distinction refers to different phases, defined by the mathematical wave equations for such orbitals. How to make molecular Orbital diagramhttps://www.youtube.com/watch?v=UYC-ndQ6Lww&t=6s Answer (1 of 8): You didn't specify the kind of theory you are considering. VB theory (Lewis dots) will give a different bond order than MO theory. In VB theory, you end up getting three bonds and a pair of electrons on one atom, C\underline{=}N: so three (Or maybe four if you put the lone pair ... Molecular orbital theory is also able to explain the presence of Figure \(\ PageIndex{6}\): Molecular Orbital Energy-Level Diagram for HCl. to describe the bonding in the cyanide ion (CN −). mix atomic orbitals on different atoms to get Molecular Orbitals. The resul7ng MO diagram looks like this. CN- (Cyanide ion), NO+ (Nitrosonium ion).
The molecular orbital configuration of C N + is K K σ (2 s) 2, σ ∗ (2 s) 2, π (2 p x ) 2, π (2 p y ) 2. Bond order is 2 . All the electrons are paired and ion is diamagnetic.
We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory.
FREE Expert Solution. We're being asked to complete the molecular orbital diagram of CN- and then determine the bond order. To do so, we shall follow these steps: Step 1: Calculate the total valence electrons present. Step 2: Fill the molecular orbitals with electrons. Step 3: Determine the bond order. Step 1: Calculate the total valence ...
Molecular orbital Diagram for Cn-mo diagram of cn hunt research group right you have been asked to draw the mo diagram for cn a heteronuclear diatomic don t panic take it one step at a time and you will have a plete mo diagram before you know it this is meant to be an interactive exercise so arrange for some pieces of blank paper a pencil a pen and an eraser molecular orbital theory ...
d orbitals •l = 2, so there are 2l + 1 = 5 d-orbitals per shell, enough room for 10 electrons. •This is why there are 10 elements in each row of the d-block. σ‐MOs for Octahedral Complexes 1. Point group Oh 2. The six ligands can interact with the metal in a sigma or pi fashion.
Apr 06, 2021 · Arrange the following molecular species in increasing order of stability. Answer: N 2 2-< N 2-= N 2+ < N 2. Question 48. Explain on the basis of the molecular orbital diagram why O 2 should be paramagnetic? Answer: O 2 molecule contains one unpaired electron in each of one π2p x and π2p y orbitals. Question 49. Define antibonding molecular ...
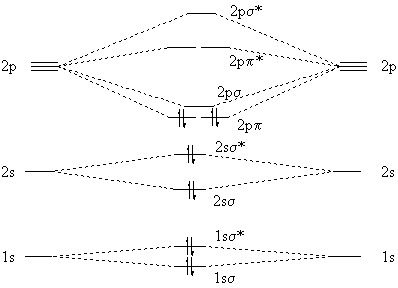
Molecular Orbitals Involving Only ns Atomic Orbitals. We begin our discussion of molecular orbitals with the simplest molecule, H 2, formed from two isolated hydrogen atoms, each with a 1s 1 electron configuration. As discussed previously, electrons can behave like waves.In the molecular orbital approach, the overlapping atomic orbitals are described by mathematical equations called wave ...
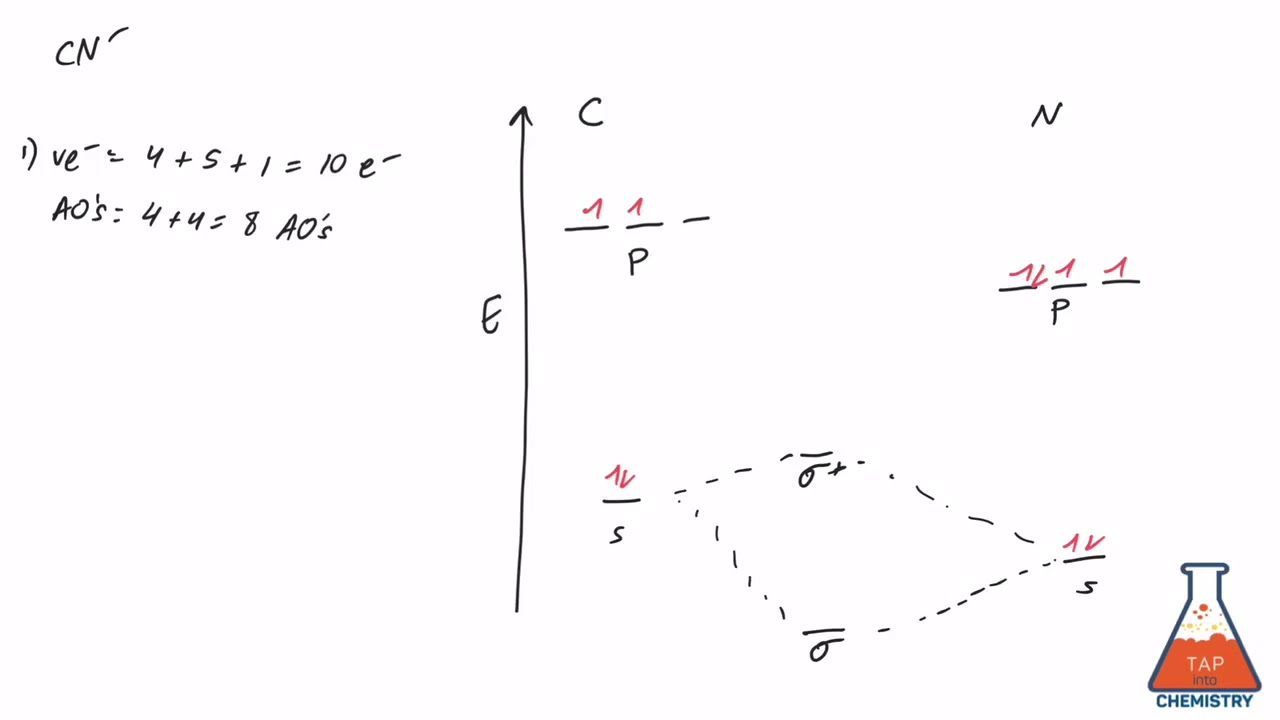
Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. Clearly, CN is hetero orbital. 3.
(b) If you combine two atomic orbitals on one atom to make a new orbital, is this a hybrid orbital or a molecular orbital? (c) Does the Pauli exclusion principle (Section 6.7) apply to MOs? Explain. _____ 9.73 Consider the H 2 + ion. (a) Sketch the molecular orbitals of the ion and draw its energy-level diagram. (b) How many electrons are there ...
Oct 25, 2021 · The superconducting phase diagram for a quasi-two-dimensional organic superconductor, κ-(BEDT-TTF)2Cu[N(CN)2]Br, was studied using pulsed magnetic field penetration depth measurements under rotating magnetic fields. At low temperatures, Hc2 was abruptly suppressed even by small tilts of the applied fields owing to the orbital pair-breaking effect. In magnetic fields parallel to the conducting ...
Molecular Orbital Mixing. More detail was added to this answer in response to input and questions from students in the class of 2008. If you have suggestions or contributions please e-mail me. First of all while the stage 1 (pre mixing diagram) of diatomics is very easy to produce, the mixing in diatomics is very difficult to evaluate because ...
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
CN Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. CN is known as cyanide which exists as a pseudohalide anion. It belongs to the cyano group and consists of carbon and a nitrogen atom having a triple bond. It carries a charge of -1 and is a conjugate base of hydrogen cyanide (HCN).
The molecular orbital energy level diagram provided shows the energies of the orbitals for the valence electrons in the free radical CN. Indicate on this diagram the ground state electronic configuration of CN using the arrow notation for electron spins. * C has 4 valence electrons and N has 5 valence electrons, giving a total of 9
Cyanide Molecular Orbital Diagram. MO Theory: the bonding orbital will be lower in energy, the an7bonding The resul7ng MO diagram looks like this. CN- (Cyanide ion), NO+ (Nitrosonium ion ). The molecular orbital diagram of (if order of molecular orbital is like that in) is as shown below. We must remember that total number of electrons in ...
longest bond (127 pm), two electrons in antibonding orbitals. 5.8 a. The CN. – energy level diagram is similar to that of NO (Problem 5.7) without the.29 pages
Feb 03, 2021 · A simplified molecular orbital diagram for an octahedral transition metal complex showing σ−and π−interactions only. Class I : In class I complexes, the Δ o splitting is small and often applies to 3d metals and σ ligands at lower end of the spectrochemical series.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
This video is about MO Diagram #3 - CN-
Molecular orbital Diagram Cn-mo diagram of cn hunt research group right you have been asked to draw the mo diagram for cn a heteronuclear diatomic don t panic take it one step at a time and you will have a plete mo diagram before you know it this is meant to be an interactive exercise so arrange for some pieces of blank paper a pencil a pen and an eraser molecular orbital theory heteronuclear ...
Walsh diagram OH 2, SH 2, NH 2 8 Bent-, FH2 + NH 2, PH 2, CH 2 7 Bent-, OH2 + CH 2, SiH 2, BH 2 6 Bent-, NH2 + BH 2, AlH 2, CH 2 5 Bent BeH 2, BH 2+ 4 Linear LiH 2, BeH 2+ 3 Linear LiH 2+ 2 Bent No. of Shape valence electrons Molecular species Known shape of some AH 2 molecules Recall: a molecule adopts the structure that best stabilises the HOMO.
The hybrid orbitals are more prominent outward so that their ability to overlap is stronger than that of normal orbitals. Molecular Formula: A chemical formula is a brief way of expressing the number and type of atoms that make up a particular chemical compound.
















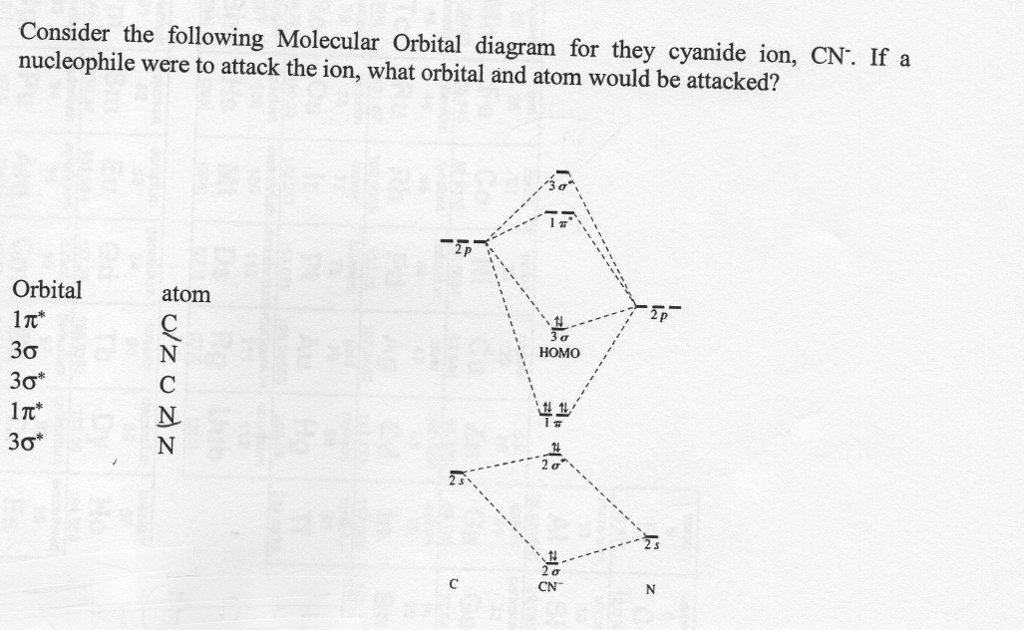


0 Response to "40 cn molecular orbital diagram"
Post a Comment