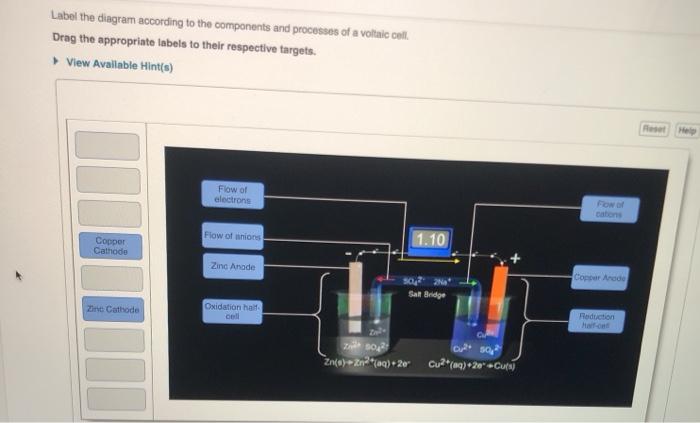
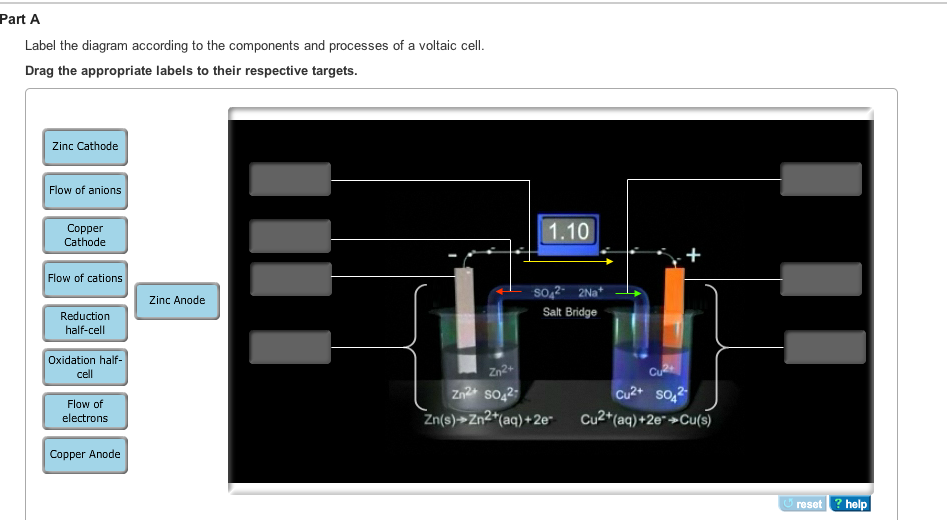
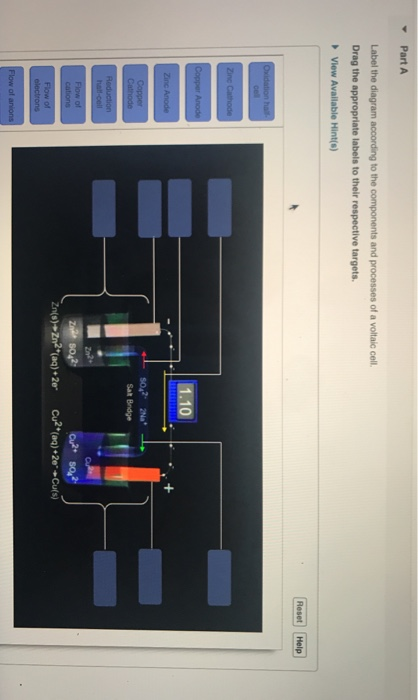
40 label the diagram according to the components and processes of a voltaic cell
Solved Label the diagram according to the components and | Chegg.com. Science. Chemistry. Chemistry questions and answers. Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate labels to their respective targets. Question: Label the diagram according to the components and processes of a voltaic cell.
A voltaic cell is made from magnesium and iron half-cells. Magnesium is a more reactive metal than iron. Which statement is correct when the cell produces electricity? A. Electrons are lost from Magnesium atoms. B. The concentration of Fe 2+ ions increases. C. Electrons flow from the iron half-cell to the Magnesium half-cell.
The animal cell diagram is widely asked in Class 10 and 12 examinations and is beneficial to understand the structure and functions of an animal. A brief explanation of the different parts of an animal cell along with a well-labelled diagram is mentioned below for reference.
Label the diagram according to the components and processes of a voltaic cell
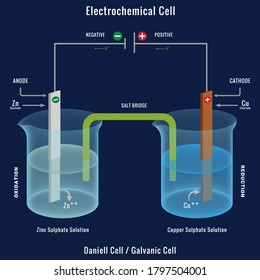
Galvanic Cell or Voltaic Cell - A Galvanic cell, also known as the Voltaic cell is a device in which electrical current is generated by a spontaneous redox reaction. A galvanic cell has two half cells. Click here for detailed content on the Galvanic cell (Voltaic Cell).
Wikipedia sayings about label the diagram according to the components and processes of a voltaic cell. drag the appropriate labels to their respective targets. 1.Glossary of engineering. inventor of the voltaic pile, the first electrical battery. In common usage, the word "battery" has come to include a single galvanic cell, but a battery
Figure 2.9 shows a two-dimensional drawing of an animal cell. The diagram shows the structures visible within a cell at high magnification. The structures form the ultrastructure of the cell. Figure 2.9: Diagram of the cell ultrastructure of an animal cell. In pairs, discuss the different organs in the human body and the way in which they function.
Label the diagram according to the components and processes of a voltaic cell.
CH 142 Spring 2012 4 2. For the following voltaic cell Pb !Pb+2 (1 M) !! Cu+2 (1 M) !Cu for which the following data were obtained, determine ΔG at each temperature, then graph ΔG versus absolute T. Find the ΔH value for this battery and the ΔS value using the graph created. Note that while there can be several values for ΔG there is just one value for ΔH and
The plasma membrane, also known as the cell surface membrane or plasmalemma, defines the boundary of the cell. It is a phospholipid bilayer with embedded proteins that encloses every living cell. It regulates the movement of materials into and out of the cell and facilitates electrical signaling between them.
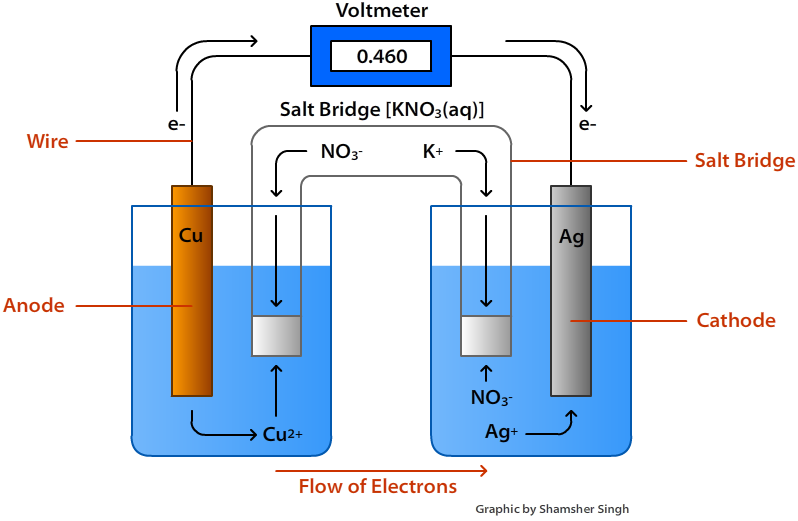
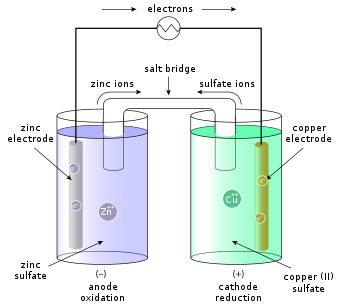
Label the diagram according to the components and processes of a voltaic cell. The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reaction and the other half-cell is the site of the reduction reaction. Type the half-cell reaction that takes place at the anode for the cobalt-silver voltaic cell.
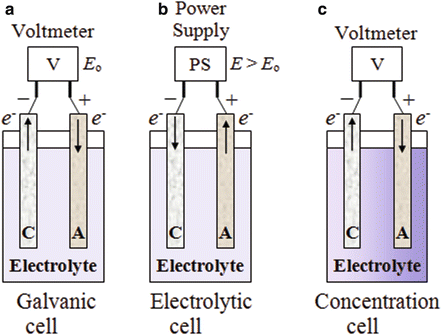
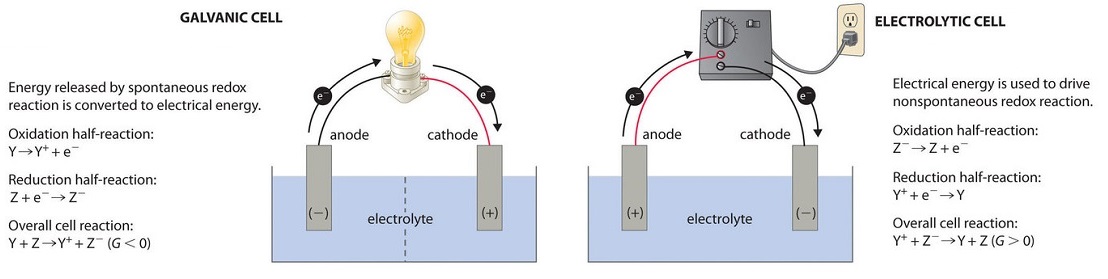
Electrolytic Cells. Voltaic cells use a spontaneous chemical reaction to drive an electric current through an external circuit. These cells are important because they are the basis for the batteries that fuel modern society. But they aren't the only kind of electrochemical cell. It is also possible to construct a cell that does work on a ...
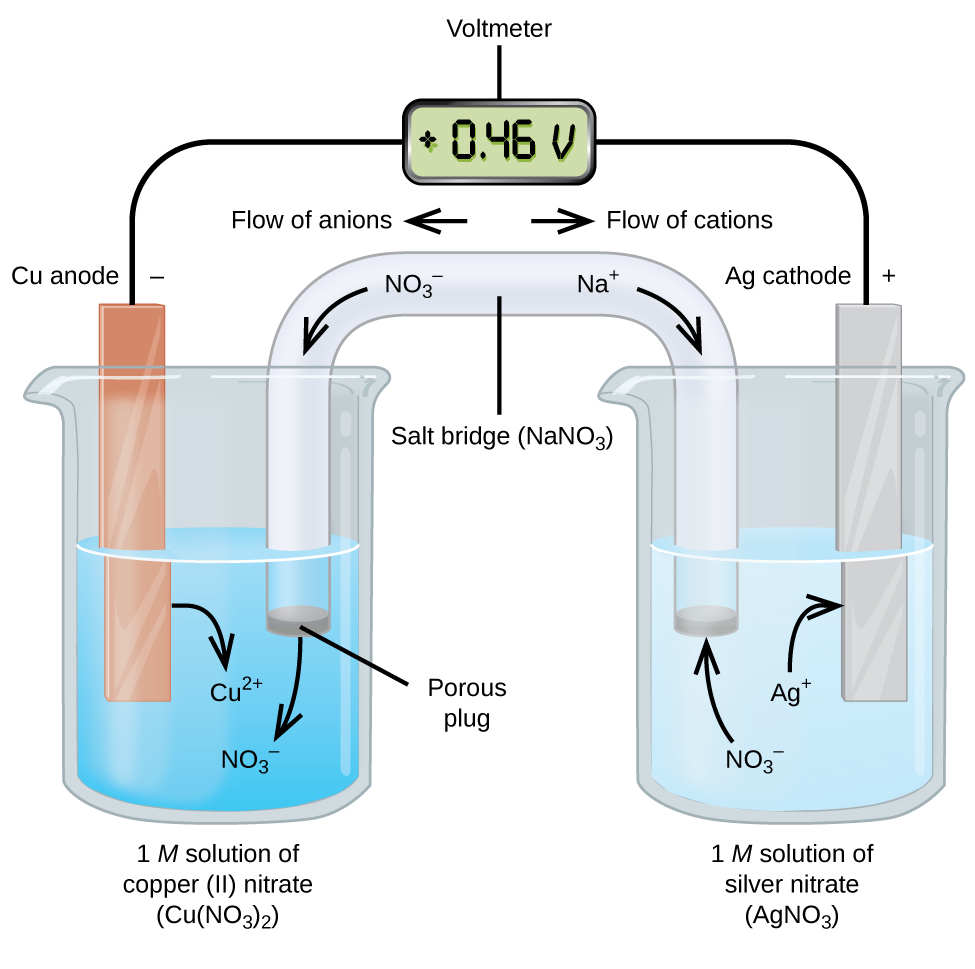
A galvanic cell based on the spontaneous reaction between copper and silver (I) is depicted in Figure 17.3. The cell is comprised of two half-cells, each containing the redox conjugate pair ("couple") of a single reactant. The half-cell shown at the left contains the Cu (0)/Cu (II) couple in the form of a solid copper foil and an aqueous ...
Question:Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate labels to their respective targets. This problem has been solved! See the answerSee the answerSee the answerdone loading thanks in advance Show transcribed image text Best Answer This is the best answer based on feedback and ratings.
Label the diagram according to the components and processes of a voltaic cell. This creates a current of electrons moving across a wire that can be used for energy. Electrolytes containing the ions involved in the redox reaction. Type the half cell reaction that takes place at the anode for the cobalt silver voltaic cell.
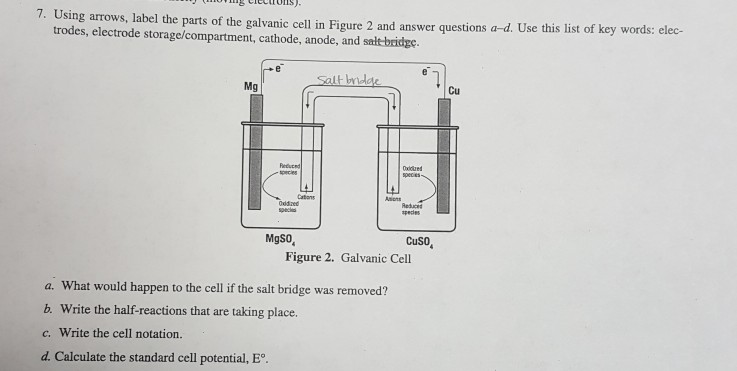
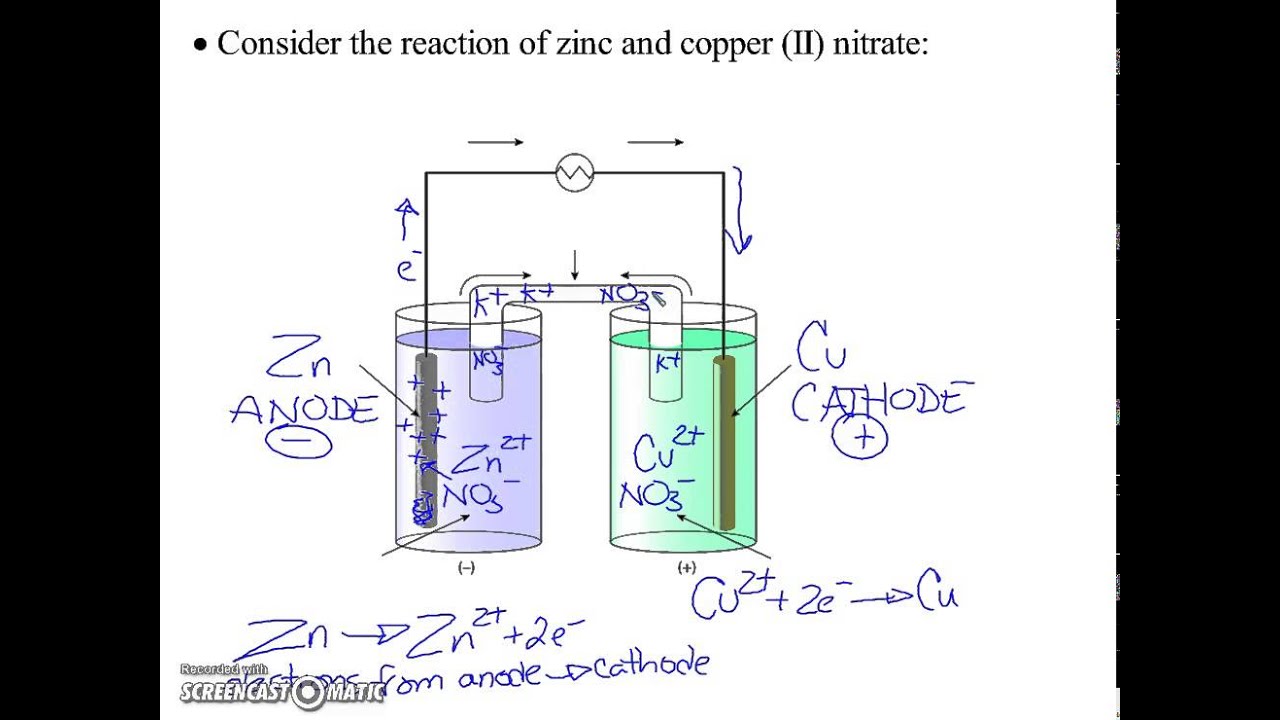
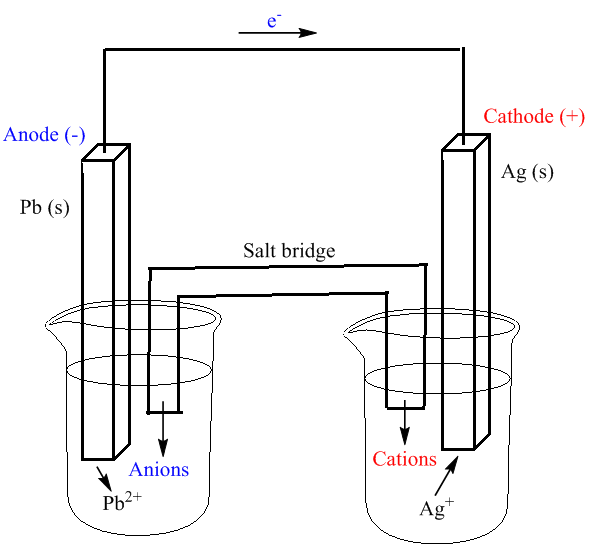
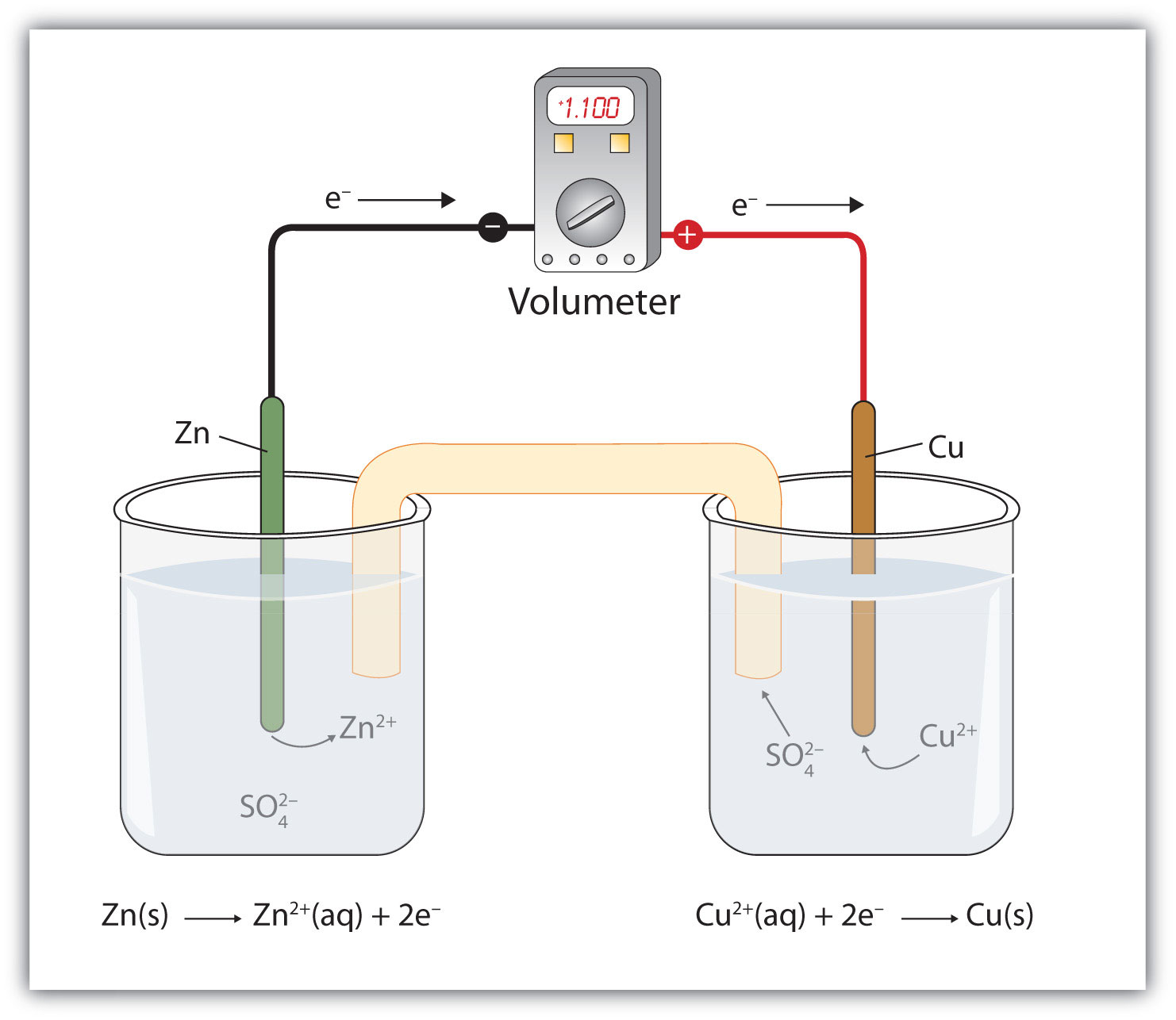
Sketch a cell diagram for the reaction. Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s) Solution: A. Draw one of the diagrams above (no labels). B. Identify what is oxidized and reduced. Zn is oxidized; Cu²⁺ is reduced. C. Put the oxidation materials in the left hand cell. Label the Zn as the negative anode. In the solution put Zn²⁺ and NO₃⁻.
Galvanic cells, also known as voltaic cells, are electrochemical cells in which spontaneous oxidation-reduction reactions produce electrical energy.In writing the equations, it is often convenient to separate the oxidation-reduction reactions into half-reactions to facilitate balancing the overall equation and to emphasize the actual chemical transformations.
Draw a diagram for this Galvanic cell, labeling the electron flow, the anode and cathode, and the positive and negative sides of the Galvanic cell. ... ("An")"ode"# of a cell, voltaic or electrochemical, attracts anions (ions with negative charges) and thus must carries some positive charges. So it is losing electrons and thus undergoing oxidation.
A voltaic cell is constructed using electrodes based on the following half reactions: a. b. Pb (aq) + Pb(s) E +3e- -> Au(sO I 52 Which is the anode and which is the cathode in this cell? P b What is the standard cell potential? +1152v + 11125 v Calculate the standard cell otential produced by a voltaic cell consisting of a nickel electrode in
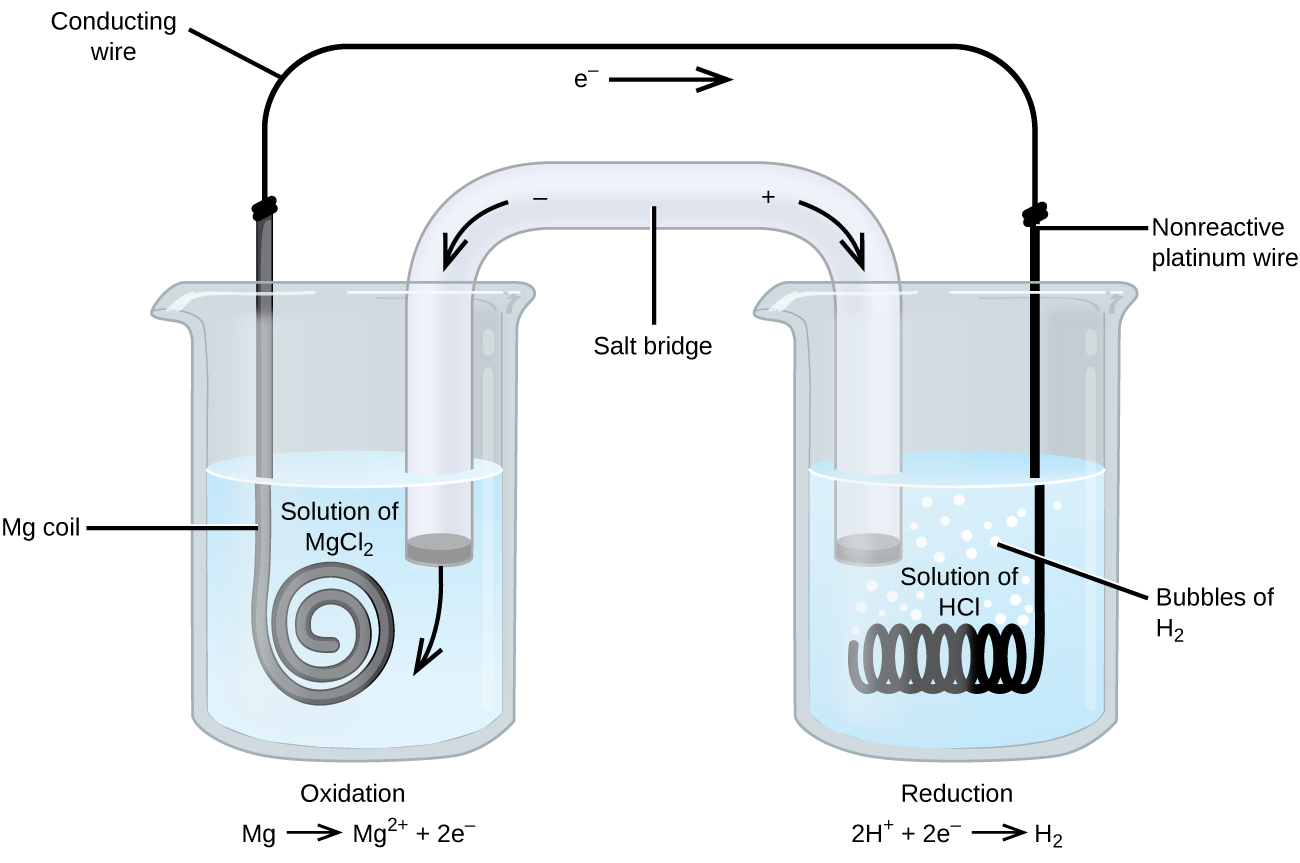
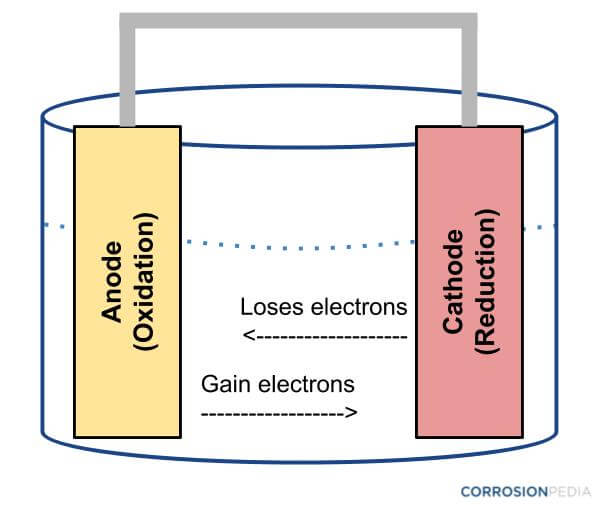
The cell as shown is a voltaic cell. A voltaic cell produces electrical energy by a spontaneous chemical reaction. Oxidation occurs at the anode and reduction occur at the cathode. The electrode at the left hand side the anode where oxidation occurs while the electrode at the right hand side is the cathode where reduction occurs.
Label the diagram according to the components and processes. This creates a current of electrons moving across a wire that can be used for energy. Answer to label the diagram according to the components and processes of a voltaic cell. A voltaic cell has several components o o o o o o anode.
Chapter 17: Electrochemistry. Label the diagram according to the components and processes of a voltaic cell. The half-cell is a chamber in the voltaic cell where one half-cell is the site of the oxidation reaction and the other half-cell is the site of the reduction reaction. Type the half-cell reaction that takes place at the anode for the ...
Basic voltaic cell diagram. In order to draw any voltaic cell, we need each of these elements in the basic diagram. We will need to know what goes into the oxidizing side and on the reducing side ...
Carlotta makes a graphic organizer about the steps of the Sun's hydrogen fusion process. A venn diagram of 3 intersecting circles with one circle labeled step 1, a second circle labeled step 2 and the third labeled step 3. There is a V in the step 1 circle. There is a Z in the step 3 circle.
Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate label to their respective targets. Learn this topic by watching Galvanic Cell Concept Videos
A voltaic cell stable enough to be used as a battery is called a Daniell cell. For our purposes, we will work with the idealized Daniell cell in the figure below. We can use the known values of the standard-state reduction potentials for the Cu/Cu 2+ and Zn/Zn 2+ half-cells to predict the overall potential for the Daniell cell and to determine ...
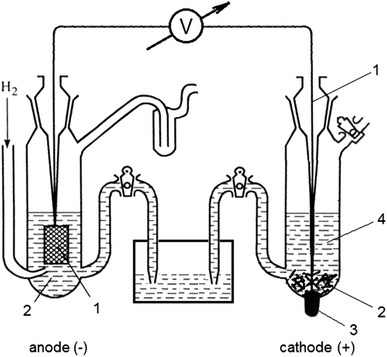
Asked for: cell diagram. Strategy: Using the symbols described, write the cell diagram beginning with the oxidation half-reaction on the left. Solution: The anode is the tin strip, and the cathode is the Pt electrode. Beginning on the left with the anode, we indicate the phase boundary between the electrode and the tin solution by a vertical bar.
Cell Organelles Definition. Cell organelles are specialized entities present inside a particular type of cell that performs a specific function. There are various cell organelles, out of which, some are common in most types of cells like cell membranes, nucleus, and cytoplasm. However, some organelles are specific to one particular type of cell ...
A galvanic cell based on the spontaneous reaction between copper and silver (I) is depicted in (Figure). The cell is comprised of two half-cells, each containing the redox conjugate pair ("couple") of a single reactant. The half-cell shown at the left contains the Cu (0)/Cu (II) couple in the form of a solid copper foil and an aqueous ...
Concept. Concept: Example: Problem: Label the diagram according to the components and processes of a voltaic cell. Drag the appropriate labels to their respective targets. FREE Expert Solution. 80% (385 ratings) play-rounded-fill. play-rounded-outline.
Label the diagram according to the components and processes of a voltaic cell. The electrode at which oxidation occurs electrons leave the anode cathode. Anode the electrode at which oxidation occurs. Electrolytes containing the ions involved in the redox reaction. The other is a copper metal strip in a solution of a copper salt.

Label the diagram according to the components and processes of a voltaic cell. drag the appropriate labels to their respective targets.

















0 Response to "40 label the diagram according to the components and processes of a voltaic cell"
Post a Comment