36 orbital diagram of fe
2. Molecular orbital theory: This is the best model to explain the bonding within the CO ligand as well as in metal carbonyl complexes. There are total three molecular diagrams for carbonyl ligand which were proposed from time to time. Though, all three molecular orbital (MO) diagrams are able to explain the nature of metal-4 The complex ion [Fe (CN)6]3- has an octahedral geometry owing to the six cyano ligands coordinated to Fe. Since CN- is a strong field ligand, there is pairing of electrons on the sub orbitals of 3d orbital hence the hybridization expected on the Fe3+ is d2sp3 (a low spin inner orbital complex ion). 1.6K views Sonali Srivastava
Nov 09, 2018 · Iron is a chemical element with symbol Fe (from Latin: ferrum) and atomic number It is a metal in the first transition schematron.org is by mass the most common element on Earth, forming much of Earth's outer and inner schematron.org is the fourth most common element in the Earth's schematron.org abundance in rocky planets like Earth is due to its abundant production by fusion in high-mass stars, where it.

Orbital diagram of fe
A schematic molecular orbital diagram of Fe (CO) 5 constructed from density-functional theory calculations. The isosurface plots of the occupied and unoccupied orbitals have been obtained with... Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau... Download scientific diagram | Qualitative valence molecular-orbital (MO) diagram of Fe(CO) 5 . Displayed is the subset of Fe(CO) 5 MOs which are derived from Fe 3d and CO 5r and 2p orbitals. For ...
Orbital diagram of fe. Fe:[Ar]3d⁵ . How many valence electrons does an arsenic atom have? ... Which principle or rule is violated by the following orbital diagram of an atom in its ground state? ** the second 2p orbital is empty. Hund's rule. The element whose atoms in the ground state have 2 half-filled orbitals is. Po. • The primary orbital interactions that form the metal‐ligand bonds in ferrocene occur between the Fed orbitalsand the ‐orbitals of the Cp ligand. • If D 5d symmetry is assumed,so that there is a centre of symmetry in the ferrocene molecule through the Fe atom there will be centro ‐symmetric (g)andanti‐ symmetric(u) combinations. Fe3+ loses an electron from the d You take electrons out of the 4s orbital, before you take them out of the 3d orbital, because it is lower.An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represe nt the electrons in each orbital. What is the orbital notation of Fe? Fe, or iron, has an atomic number of 26. notation is 1s2 2s2 2p6 3s2 3p6 4s2 3d6. What is an orbital diagram? An orbital diagram is similar to electron...
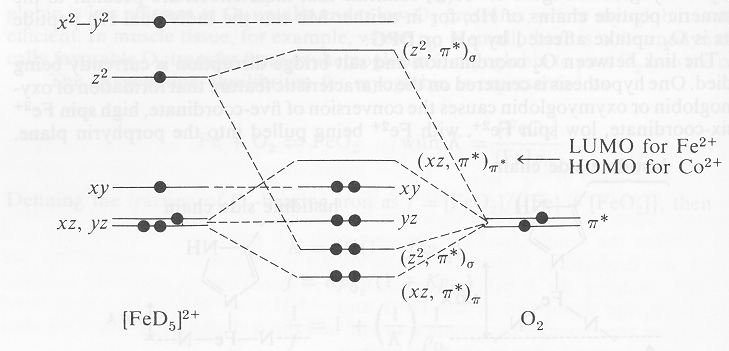
for the molecular orbitals with high d orbital character. metal d orbitals xz yz x2-y2 xy z2 1.125e 2.75e b. For consideration of L as a -acceptor in the axial position, the identical energy level diagram is obtained regardless of whether L is assigned to position 1 or 6. The xz and yz Mar 22, 2021 · What is electron configuration of Fe? [Ar] 3d6 4s2. How many d electrons are in FE? From the periodic table, iron has atomic number 26 , meaning that there are 26 electrons in each ground state iron atom. 2⋅5=10 electron in each d orbital, and so on so forth. Electron orbitals fill according to the Aufbau (Build-up) Principle. After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d9. Therefore the expected electron configuration for Copper will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 9 . Note that when writing the electron configuration for an atom like Cu, the 3d is usually written before the 4s. 23 Jun 2016 — 1 Answer · Its core orbitals are the 1s , 2s , 2p 's, 3s , and 3p 's. · Its valence orbitals are the 4s and 3d 's.1 answer · Iron is on the fourth row of the periodic table, sixth column of the transition metals, atomic number 26. What we have is: • Its core orbitals are ...
Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ... Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions) In writing the electron configuration for Iron the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Iron go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.electron configuration for Fe2+ - CHEMISTRY COMMUNITYMolecular orbital diagram - Wikipedia Orbital Notation for Iron (Fe). Mr. Causey shows you step by step how to write the orbital notation for iron (Fe).http://yourCHEMcoach.comSUBSCRIBE for more ...
Write the complete orbital configuration for iron (Fe). Orbital Configuration: Orbital configuration is the arrangement of electrons present in the atomic structure.
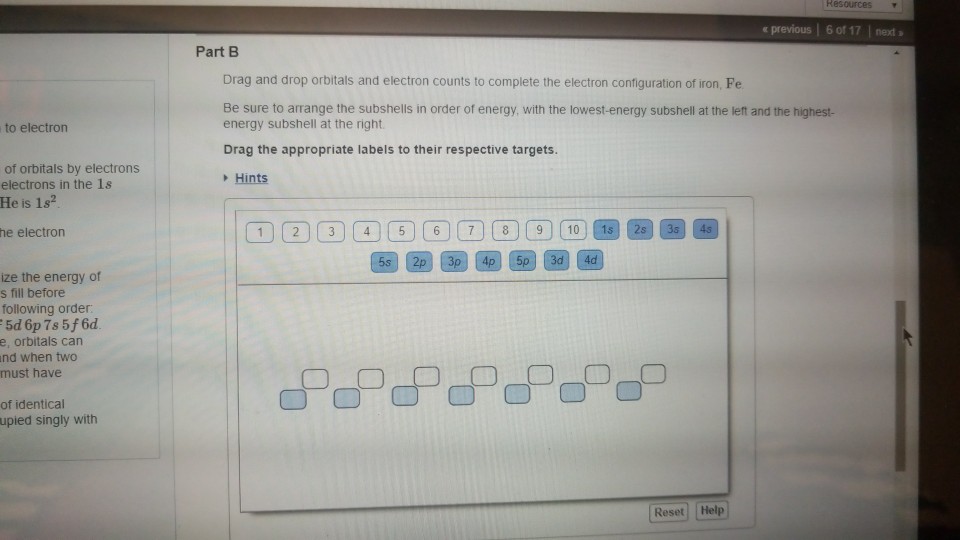
Iron has 26 electrons so its normal electron configuration would be: Fe 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. When we make a 3+ ion for Iron, ... Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes":
The overall molecular orbital energy level diagram for σ-bonding in octahedral complexes can be shown as: Figure 10. The formation of σ-molecular orbitals (bonding, antibonding and non-bonding) in octahedral complexes of transition metals. Buy the complete book with TOC navigation,
Example: Constructing MOs for Titanium Tetraiso propoxide, Ti(OiPr) pp , 4 • The OiiPrSALCs are compridised of fill dfilled p d bit l th O t x an p y orbitals on e a oms. • Ti bonding AOs E: (3dz 2, 3dx2‐y ) T 2: (4p x , 4p y, 4p z) (3dxy, 3dxz, 3dyz) • The T 1 SALC is non‐bonding. • Significant overlap occurs between the E SALC 2and the e AOs on Ti (3dz2, 3dx2‐y )
Iron (Fe) has an atomic mass of 26. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
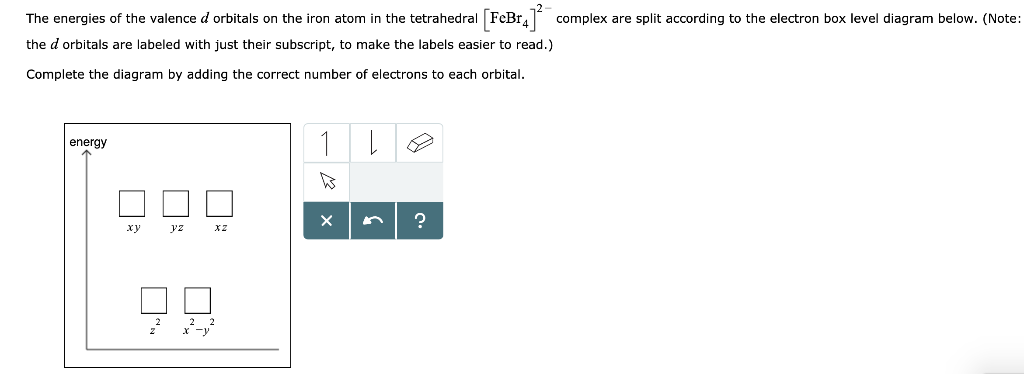
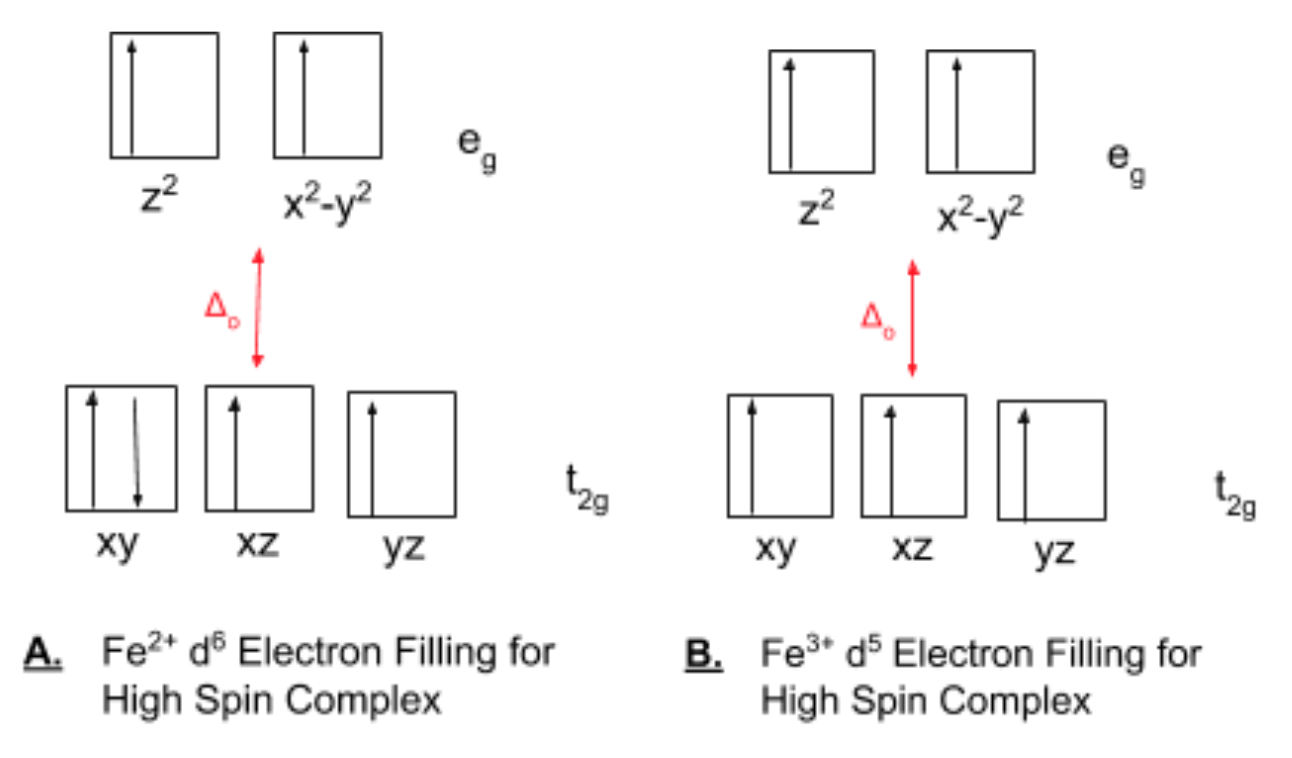
d-orbital diagram for [Fe(H 2 O) 6] 3+: The first three electrons go into t 2g orbitals unpaired. The 4th and 5th electrons must choose whether to pair up with electrons already in t 2g (which costs energy) or to go into higher energy e g orbitals (which also costs energy). In this case, the splitting energy is less than the pairing energy so the 4th and 5th electrons go into the e g orbitals.
The electron configuration of iron shows that the last shell of iron (Fe) has two (4s 2) electrons and the d-orbital has a total of six electrons (3d 6 ). Therefore, the valence electrons of iron (Fe) are eight. In this way, the valence electrons of all the transition elements can be determined.
Orbital diagrams are a simple way of showing the way the electrons are arranged within an atom or molecule. They are constructed by sequential orbitals from low ...1 answer · Top answer: Iron orbital diagram Iron is in the d-block in the fourth period of the periodic table. This means that the valence electrons are in the 3d...
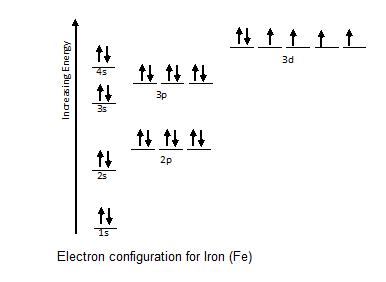
ORBITAL DIAGRAM Y ↿⇂ 1s ↿⇂ 2s ↿ ↿ ↿ 2p N Fill orbitals with "up" before "down" to maximize number of unpaired electrons. ↿⇂ 1s ↿⇂ 2s ↿⇂ ↿⇂ ↿⇂ 2p Na ↿⇂ 3s ↿⇂ 3s Fe ↿⇂ ↿⇂ ↿⇂ 3p ↿⇂ 4s ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 3d Let's only worry about the outermost orbitals for Fe.
Atomic Orbital Diagram for Iron(Fe) Iron ion(Fe 2+,Fe 3+) electron configuration. Ground state electron configuration of iron(Fe) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. The electron configuration shows that the last shell of iron has two electrons and the d-orbital has a total of six electrons. In this case, the valence electrons of iron are eight. There are two types of iron ions.
Iron (Fe) is a transition metal that follows the Aufbau rule of the filling of atomic orbitals. The atomic number of Fe is 26, which means that its atoms contain 26 protons in their nuclei, and if neutral, 26 electrons in their electron clouds. The ground state electron configuration of Fe is: 1s22s22p63s23p63d64s2
Orbital Diagram For Fe3+. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). Fe3+ loses an electron from the d You take electrons out of the 4s orbital, before you take them out of the 3d orbital, because it is lower. -3,-2,-1,0,1,2,3 c.
Download scientific diagram | Qualitative valence molecular-orbital (MO) diagram of Fe(CO) 5 . Displayed is the subset of Fe(CO) 5 MOs which are derived from Fe 3d and CO 5r and 2p orbitals. For ...
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
A schematic molecular orbital diagram of Fe (CO) 5 constructed from density-functional theory calculations. The isosurface plots of the occupied and unoccupied orbitals have been obtained with...





















.png)
complexes.png?revision=1&size=bestfit&width=545&height=372)
![Electron orbitals [2010-12-15] | Electrons, Awkward, Rearrange](https://i.pinimg.com/originals/89/ab/8f/89ab8fae9b13b38e354593c867394935.png)

0 Response to "36 orbital diagram of fe"
Post a Comment