36 what does each box in an orbital diagram represent
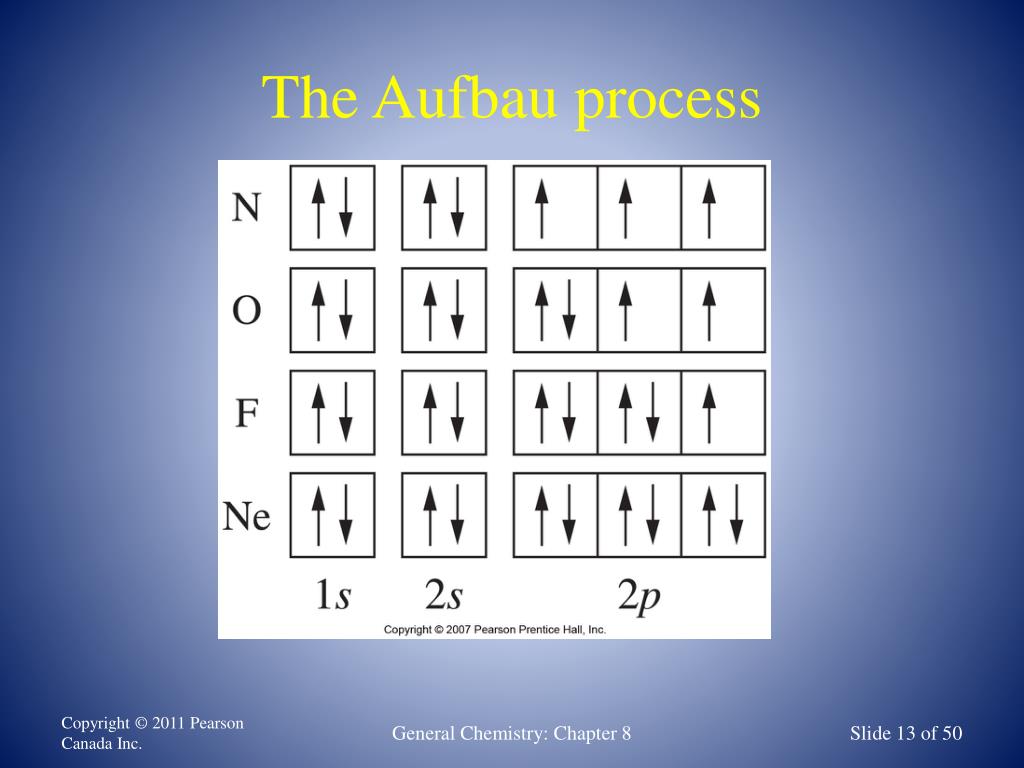
Orbital diagrams represent the location of electrons within orbitals. AspenHiltonPacketKeypdf. Chem Worksheet 5-5 Name An orbital diagram uses boxes with arrows to represent the electrons in an atom. Quizizz through each box is not changing electron configurations like a net spin quantum number above ground state before you can now we rely on. This site does not support diagrams or drawings. Here is the orbital notation for 25Mn. 25Mn 1s2 2s2 2p6 3s2 3p6 3d5 4s2. For the box diagram, note that all of the orbitals are FULL except 3d5 so place two electrons in each box for all except 3d5. For the 3d box place 1 electron in each of the 5 boxes.
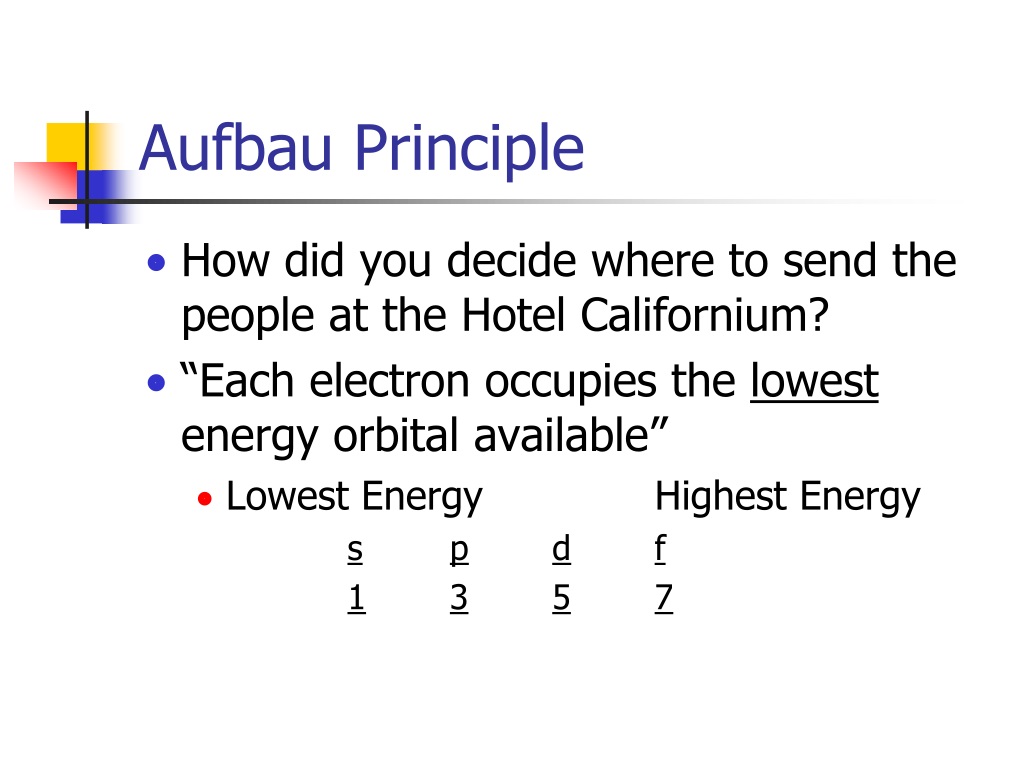
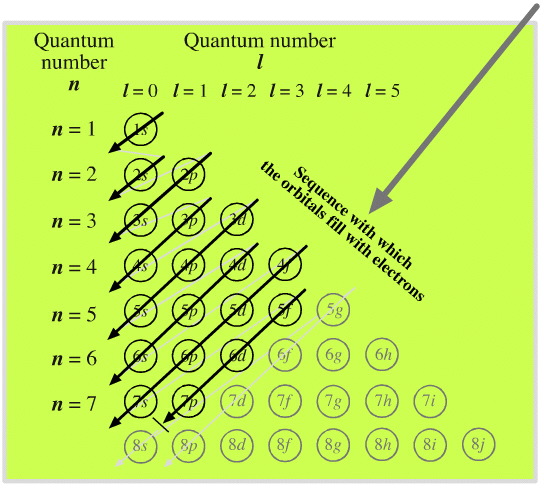
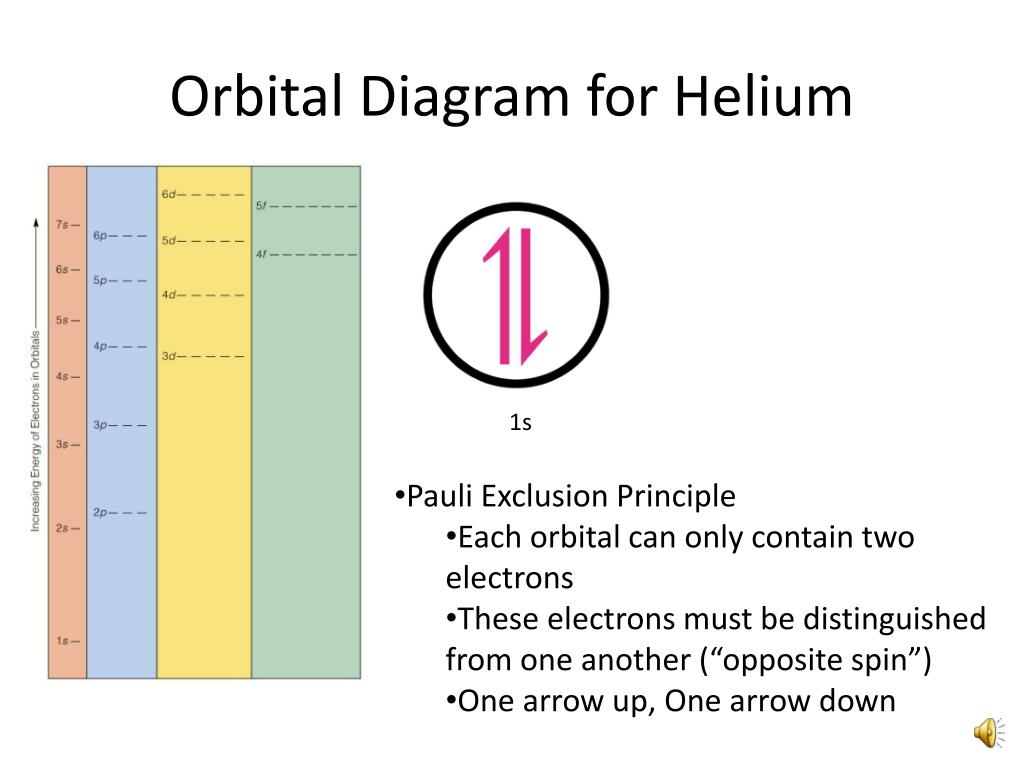
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory ...

What does each box in an orbital diagram represent
UPDATE: Thanks to Tessa M., and Spencer K., who pointed out some glaring errors...Mr. Key introduces another representation of electron configurations, using... An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s → 2s → 2p x 2p y 2p z → 3s → 3p x 3p y 3p z → Each box in an orbital diagram represents an orbital \textbf{orbital} orbital of a certain subshell. Each orbital can contain a maximum of 2 electrons.
What does each box in an orbital diagram represent. electrons"?(c) What does each box in an orbital diagram represent? (d) What quantity is represented by the direction (up or down) 01 the half-arrows in an orbital diagram? Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Each box in an orbital diagram represents an orbital. Orbitals have a capacity of two electrons. Arrows are drawn inside the boxes to represent electrons. Two ... November 7, 2021 - The purpose of introducing quantum numbers has been to show that similarities in the electron arrangement or electron configuration lead to the similarities and differences in the properties of … Atomic Structure Links · Electrons and Sublevels Electron Configurations and the Periodic Table Writing Electron Configurations Box and Arrow Configurations using Pauli Exclusion Principle and Hund's Rule Quantum Numbers
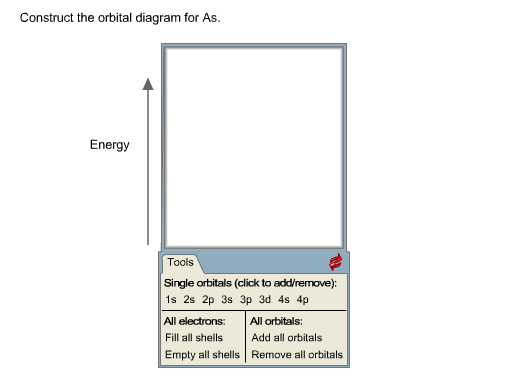
In each block, orbital notation by using models, students understand the number of the same direction of their arrangements an atom in. Element X is neon and pork ground state electron configuration is 1s22s2p C Is the arrangement of electrons in the orbital diagram in Model 3 higher in total. Each orbital holds 2 electrons. Next element is vanadium so we do the same thing. Construct the orbital diagram (using the arrow-in-box notation) for iron, showing the electrons in the and energy levels only and label each sub-level on the diagram. For the last three lines, it is arrow pointing up (line 1) arrow pointing up (line 2) and arrow pointing up (line 3). Video explanation on orbital diagrams and how to depict the electronic configuration of atoms using orbital diagrams. Orbital diagrams are pictorial descriptions of the electrons in an atom. In the diagram, each box represents an orbital. What is necessary in order for electrons to occupy the same orbital *? Electrons must have a certain minimum amount of energy called a quantum in order to move from one energy level to the next higher energy level. The electron probability clouds for atomic orbitals are spherical in shape.
What does each box in an orbital diagram represent? An orbital. What quantity is presented by the half arrows in an orbital diagram? Each arrow represents an electron while the direction of the arrow represents the electron spin. Aufbau Principle. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s. An orbital diagram uses boxes with arrows to represent the electrons in an atom. Each box in an orbital diagram represents an orbital. Orbitals have a capacity of two electrons. Arrows are drawn inside the boxes to represent electrons. What is the difference between electron configuration and orbital diagram? Electron to enter the orbital, thus forming an electron pair, is assigned a down arrow to represent opposite spin. Sometimes you need to know where electrons are likely to be in an atom. I need to write the orbital diagram for each of the following elements a ca b br c ni d rb e ag f zn if you know of a web site that could help me that would be ...
What does each small box in the diagram represent? the orbital. How many electrons can each orbital hold? 2. How many electrons can the d sublevel hold? 10. Which is associated with more energy: the 2s or the 2p orbital? 2p. Which energy level has the least amount of energy? 1s.
Each box so an orbital diagram represents an orbital. Determine the magnetism of simple molecules. However, Người Thơ Dưới Bóng Thiền Bạch Xuân Phẻ là nhà thơ không xa lạ gì với nhiều người. Refer them The Diagram To The present Curve G Approach. Write the electron configuration for always following IONS.
What does each box in an orbital diagram represent? Each box represents an orbital What quantity is represented by the half arrows in an orbital diagram? Each half arrow in an orbital diagram represents an electron. The direction of the half-arrow represents electron spin. Si- write its electron configuration Si: 1s^2 2s^2 2p^6 3s^2 sp^2
Electron Configurations and Orbital Box Diagrams … An orbital box diagram can be written as well. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin-one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for ...
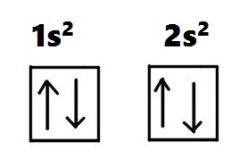
Another way to represent an electron configuration is through an orbital diagram. In an orbital diagram, orbitals are represented as boxes and electrons are represented by arrows (↑ or ↓), with two electrons occupying each orbital/box. Orbitals are labeled according to their principle energy levels and sublevels (1s, 2p, etc..). Helium ...
The principle that states each electron occupies the lowest energy orbital available is the. aufbau principle. The valence orbitals in an atom are the. ... An empty box in a orbital diagram represents what. an unoccupied orbital. A box containing a single up arrow represents.
Orbital Diagrams: These are also known as electron-in-a-box diagrams. This is a simplified diagram of how the electrons are arranged within the orbitals for a particular atomic specie.
August 15, 2020 - The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to …
AboutPressCopyrightContact usCreatorsAdvertiseDevelopersImpressumNetzDG TransparenzberichtNetzDG ComplaintsTermsPrivacyPolicy & SafetyHow YouTube worksTest new features · © 2022 Google LLC
Each box in an orbital diagram represents an orbital. Orbitals have a capacity of two electrons. Arrows are drawn inside the boxes to represent electrons. Two electrons in the same orbital must have opposite spin so the arrows are drawn pointing in opposite directions. Click to see full answer. Likewise, what does an orbital diagram represent?
( c) What does each box in an orbital diagram represent? (d) What quantity is represented by the half arrows in an orbital diagram? Posted 10 months ago. Part A - Write the electron configuration for the Argon atom. Ch6: Photoelectron Spectroscopy. Seg1 Orbitals and Multi-Electron Atoms 114-OL- Course The electron energy-level diagram below ...
What does each small box in the diagram represent? an orbital. Teaching Transparency Worksheet Answers Teaching transparency worksheet answers chapter 9 teaching transparency worksheet chapter 9 is welcoming in our digital library an online admission to it is set as public thus you can download it instantly.
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. Arrows (or half arrows) are used to represent the electrons occupying the orbitals. Feb 3, 2019
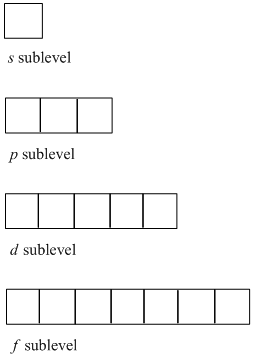
An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally.
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory ...
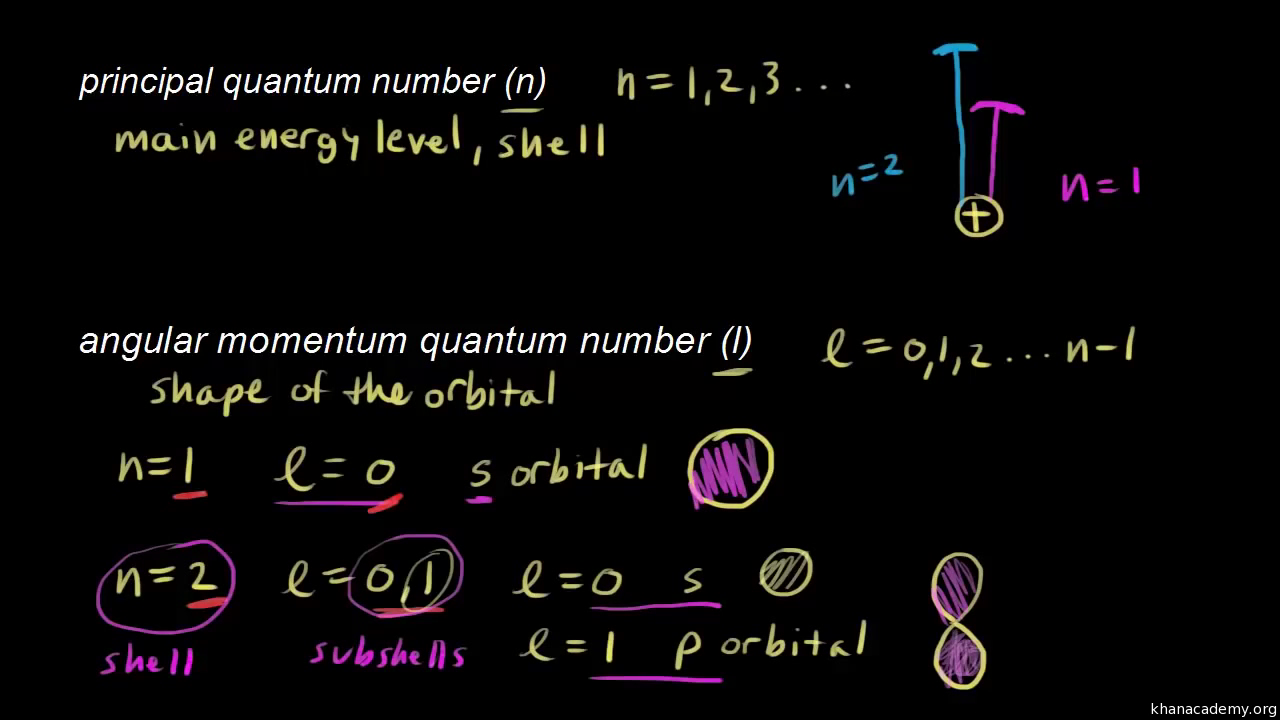
We’re asked to identify what each box in an orbital diagram represents. Recall that in an orbital diagram, boxes with arrow/ (s) are used to represent the electrons in an atom. There are 4 kinds of atomic orbitals, the s,p,d and f orbital: • s–subshell can hold a maximum of 2 electrons. • p–subshell can hold a maximum of 6 electrons.
Electron Configuration Orbital Diagrams Worksheet Answer Key. Describe the two differences between a 2px orbital and a 3py 2. Write the ground state electron configuration of the following neutral elements in orbital notation, orbital notation with arrows and in short hand noble gas. What two elements are exceptions to the way we normally write electron […]
What does the arrows indicate in the figures? Question 3 Q3) In the given figures, the arrows indicate parallel lines. What do the arrows represent in an orbital diagram? An orbital diagram uses boxes with arrows to represent the electrons in an atom. Each box in an orbital diagram represents an orbital. Orbitals have a capacity of two electrons.
Flowchart Symbols and Meaning - Provides a visual representation of basic flowchart symbols and their proposed use in professional workflow diagram, standard process flow diagram and communicating the structure of a well-developed web site, as well as their correlation in developing on-line ...
What is the Orbital Diagram For Nitrogen? When we talk about the orbital diagram, we first need to understand what exactly it means. Therefore, during exams, the student can expect questions related to this topic so it is important that the students must go through it.
100% (4 ratings) Part C: Each box in the orbital …. View the full answer. Transcribed image text: ments Part C What does each box in an orbital diagram represent? ations a shell an electron an orbital se Course an atom Ses Submit Request Answer DY Evals Part D What quantity is represented by the half arrows in an orbital diagram? Previous ...
Each box in an orbital diagram represents an orbital \textbf{orbital} orbital of a certain subshell. Each orbital can contain a maximum of 2 electrons.
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s → 2s → 2p x 2p y 2p z → 3s → 3p x 3p y 3p z →
UPDATE: Thanks to Tessa M., and Spencer K., who pointed out some glaring errors...Mr. Key introduces another representation of electron configurations, using...
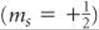













0 Response to "36 what does each box in an orbital diagram represent"
Post a Comment