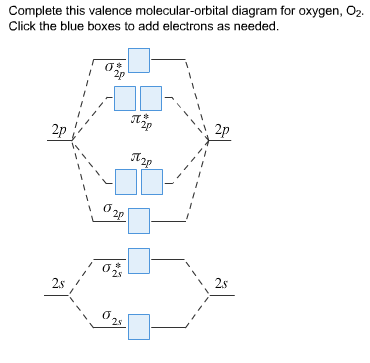
41 use the following mo diagram to find the bond order for o2.
Click to see our best Video content. Take A Sneak Peak At The Movies Coming Out This Week (8/12) Minneapolis-St. Paul Movie Theaters: A Complete Guide Unlike valence bond theory, which uses hybrid orbitals that are assigned to one specific atom, ... We can determine bond order with the following equation:.
The bond order is the number of bonds present between two atoms in a molecule or ion. Various molecular properties can be understood by this concept such as ...1 answer · Top answer: All the molecule posses bond order. The bond order varies from one molecule to another. Oxygen is a diatomic molecule. Let us first know what do you ...

Use the following mo diagram to find the bond order for o2.
Jan 31, 2018 — To find the bond order of O2+ we can use the concept of Molecular Orbital Theory. In this method we have to count the number of molecules in the Bonding ...7 answers · 57 votes: O2 2- bond order = 1 O2 - bond order = 1.5 O2 bond order = 2 O2+ bond order = 2.5 O2 ...What is the molecular orbital diagram for O2- and ...5 answersMar 27, 2017How do I find the bond order of Cl2? - Quora4 answersSep 29, 2017Using molecular orbital theory, explain why the ...3 answersMay 21, 2018NO, NO+ and NO-. Using the molecular orbital theory ...5 answersJun 9, 2018More results from www.quora.com 1 answerWe're being asked to determine the bond order and electron configuration of O2+. For this, we need to do the following steps: Step 1: Calculate the total ... Although the DTIC may or may not use these sites as additional distribution channels for Department of Defense information, it does not exercise editorial control over all of the information that you may find at these locations. Such hyperlinks are provided consistent with the stated purpose of this website.
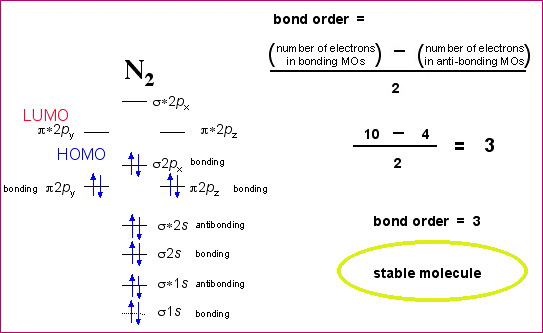
Use the following mo diagram to find the bond order for o2.. Drop all the files you want your writer to use in processing your order. If you forget to attach the files when filling the order form, you can upload them by clicking on the “files” button on your personal order page. The files should be uploaded as soon as possible to give the writer time to review and use them in processing your order. Mar 18, 2020 — Arrange the following four molecular oxygen species in order of increasing bond length: O+2, O2, O−2, and O2−2. Solution. The bond length in ... N2 has a bond order of 3 and O2 has a bond order of 2. Based on this information, choose the response that best completes the following sentence: N2 is (less, more) stable than O2, and has a (larger, shorter) bond length and a (higher, lower) bond energy. Unlike valence bond theory, which uses hybrid orbitals that are assigned to one specific atom, ... We can determine bond order with the following equation:.
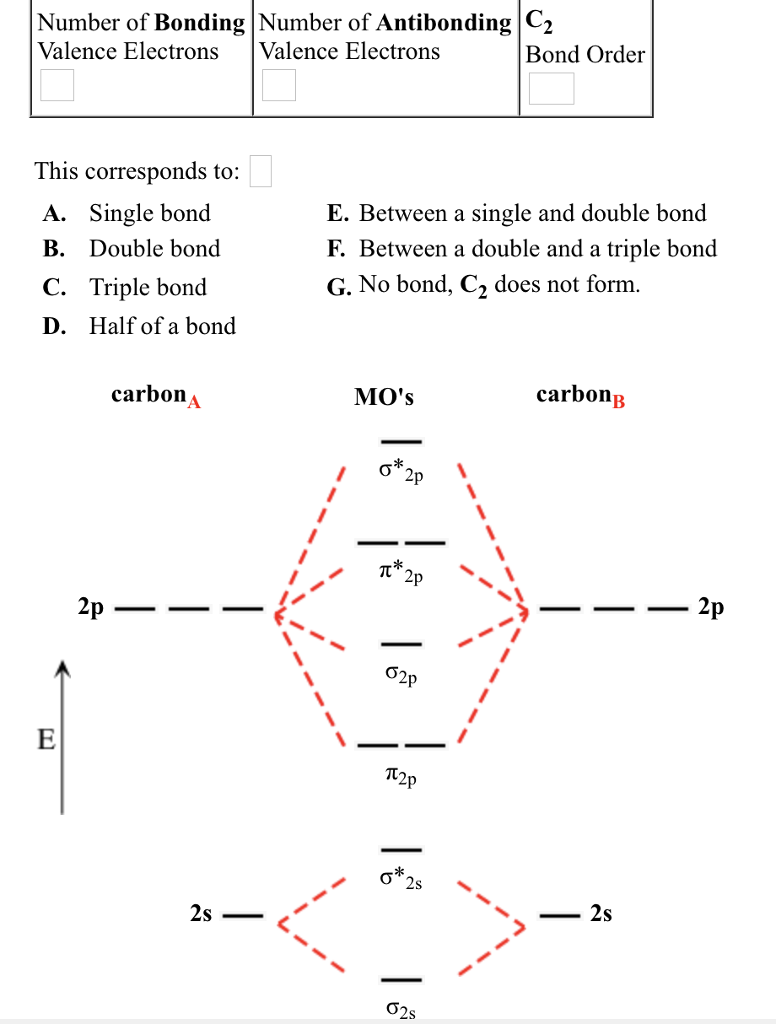
LECTURE 13. 1. Draw a molecular orbital diagram and determine the bond order expected for the molecule B2. For full credit on MO diagrams,.4 pages Academia.edu is a platform for academics to share research papers. To study a key fuel-cell reaction, a chemical engineer has 20.0-L tanks of H2 and of O2 and wants to use up both tanks to form 28.0 mol of water at $23.8 ^ { \circ } \mathrm { C }$ (a) Use the ideal gas law to find the pressure needed in each tank, (b) Use the van der Waals equation to find the pressure needed in each tank. Molecular Orbitals of the Second Energy Level, Bond Order ... can be overcome by using a more sophisticated model of bonding based on molecular orbitals.
The hydroxylating system was inhibited 49% by CO at a CO:O2 ratio of 4.0. The formation of omega-hydroxydodecanol was more sharply inhibited by CO than was the formation of (omega-1)-hydroxydodecanol, implying that more than one cytochrome P-450 was involved in the hydroxylation of 1-dodecanol and that CO has a higher affinity for the P-450 ... The red-shift within the absorption edge follows an increasing order, such as P-doped TiO 2 /MWCNTs > TiO 2 /MWCNTs > TiO 2. The following Tauc equation was used to compute the band gap of the prepared thin films: (4) αhν= (hν − Eg) 1/2. Download : Download high-res image (426KB) Download : Download full-size image; Fig. 7. The support team will view it after the order form and payment is complete and then they will find an academic writer who matches your order description perfectly. Once you submit your instructions, while your order is in progress and even after its completion, our support team will monitor it to provide you with timely assistance. EXPL THER /The purpose of this study was/ to screen for inositol-depleting valproate-like compounds as potential mood stabilizing drugs.We exploited the yeast Saccharomyces cerevisiae, as a model in which inositol de novo synthesis has been extensively characterized, to test the effects of ethyl butyrate (EB), 2-ethyl-butyric acid, sodium butyrate, and n-propyl …
Although the DTIC may or may not use these sites as additional distribution channels for Department of Defense information, it does not exercise editorial control over all of the information that you may find at these locations. Such hyperlinks are provided consistent with the stated purpose of this website.
1 answerWe're being asked to determine the bond order and electron configuration of O2+. For this, we need to do the following steps: Step 1: Calculate the total ...
Jan 31, 2018 — To find the bond order of O2+ we can use the concept of Molecular Orbital Theory. In this method we have to count the number of molecules in the Bonding ...7 answers · 57 votes: O2 2- bond order = 1 O2 - bond order = 1.5 O2 bond order = 2 O2+ bond order = 2.5 O2 ...What is the molecular orbital diagram for O2- and ...5 answersMar 27, 2017How do I find the bond order of Cl2? - Quora4 answersSep 29, 2017Using molecular orbital theory, explain why the ...3 answersMay 21, 2018NO, NO+ and NO-. Using the molecular orbital theory ...5 answersJun 9, 2018More results from www.quora.com
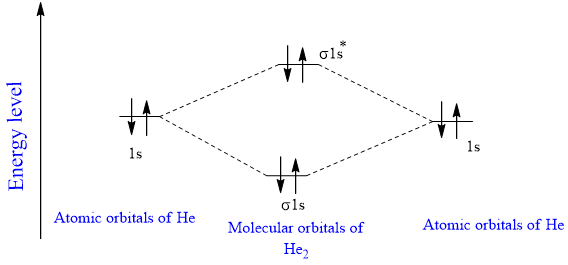
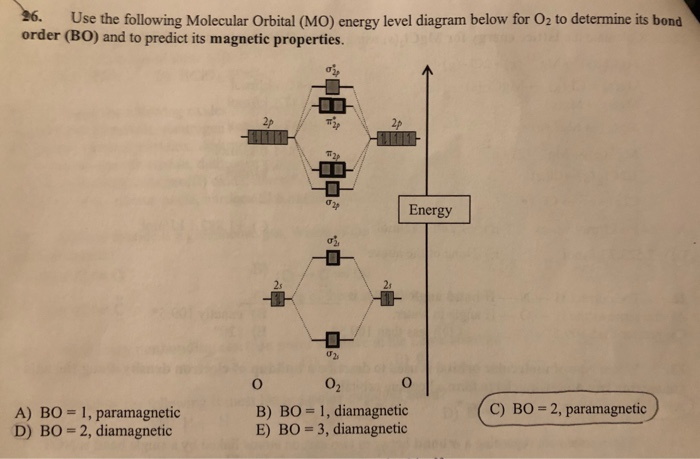


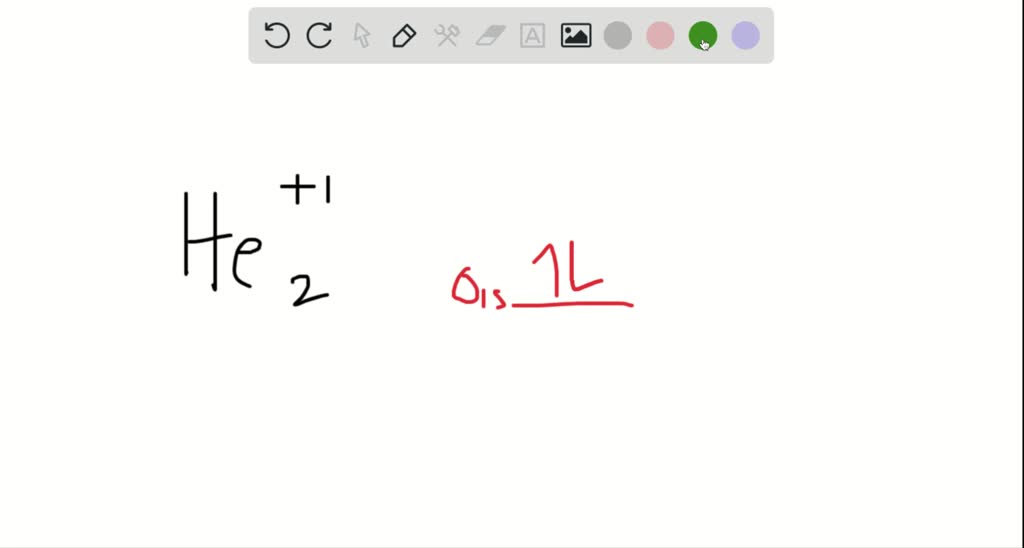
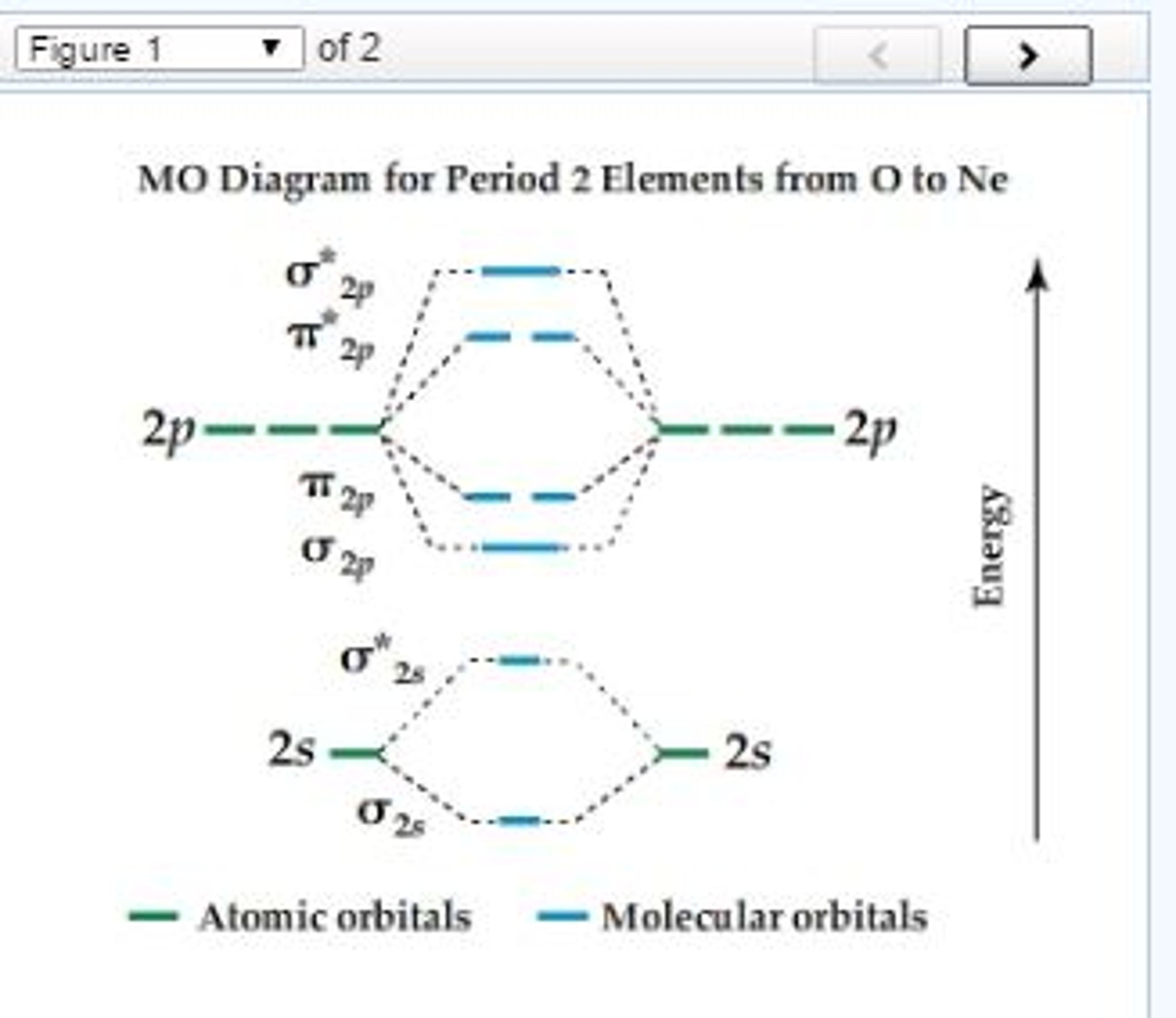










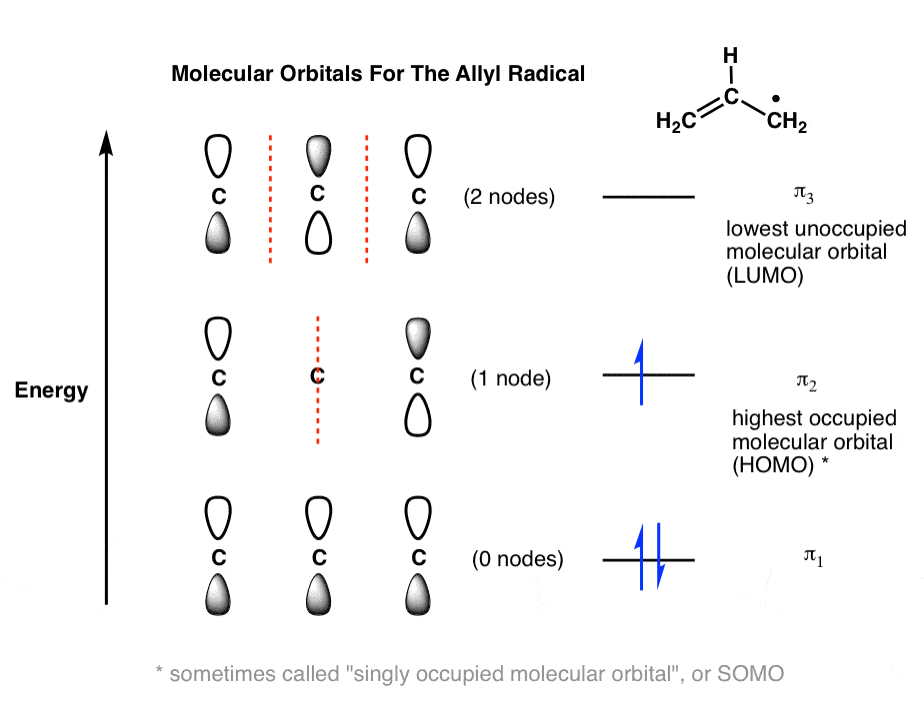


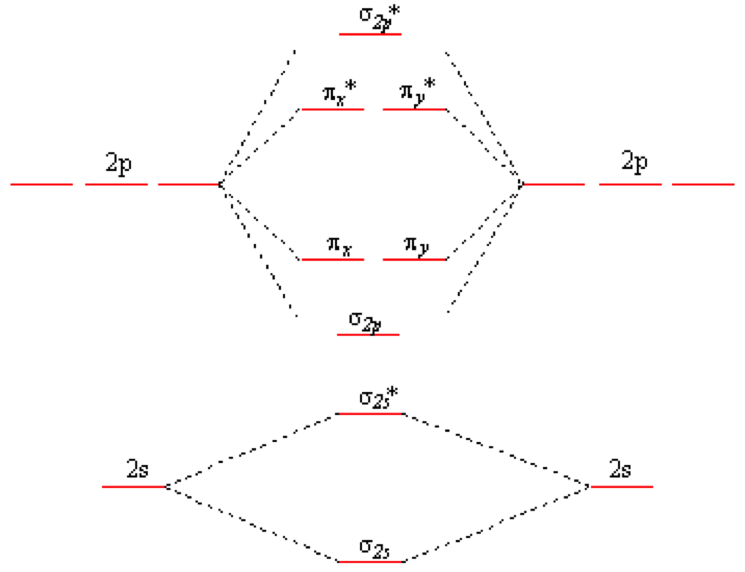
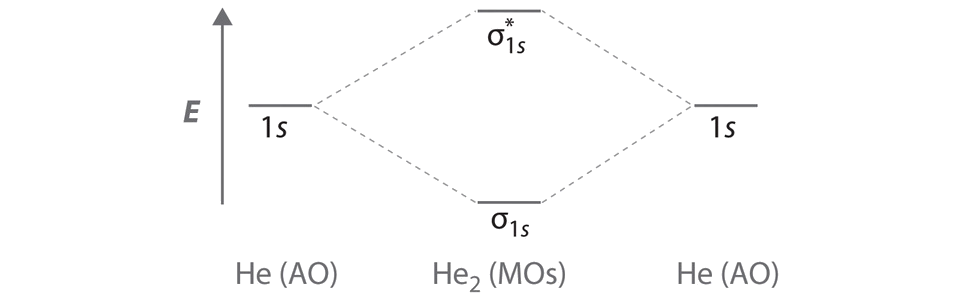
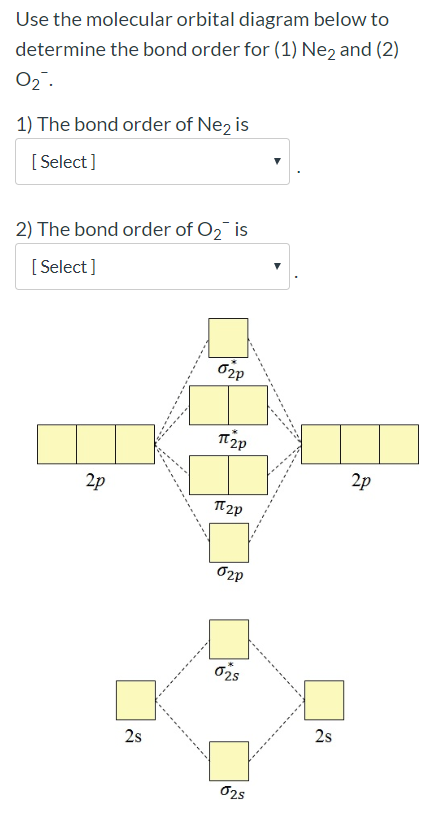



0 Response to "41 use the following mo diagram to find the bond order for o2."
Post a Comment