36 salt water phase diagram
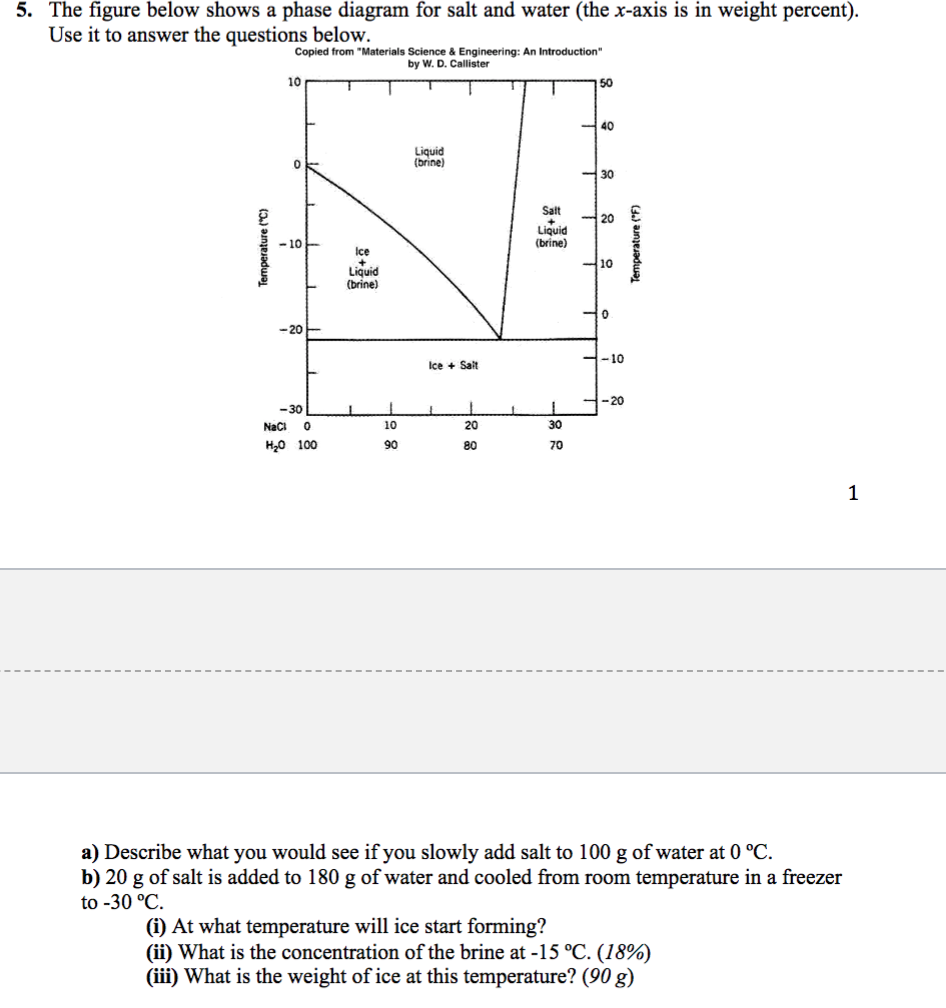
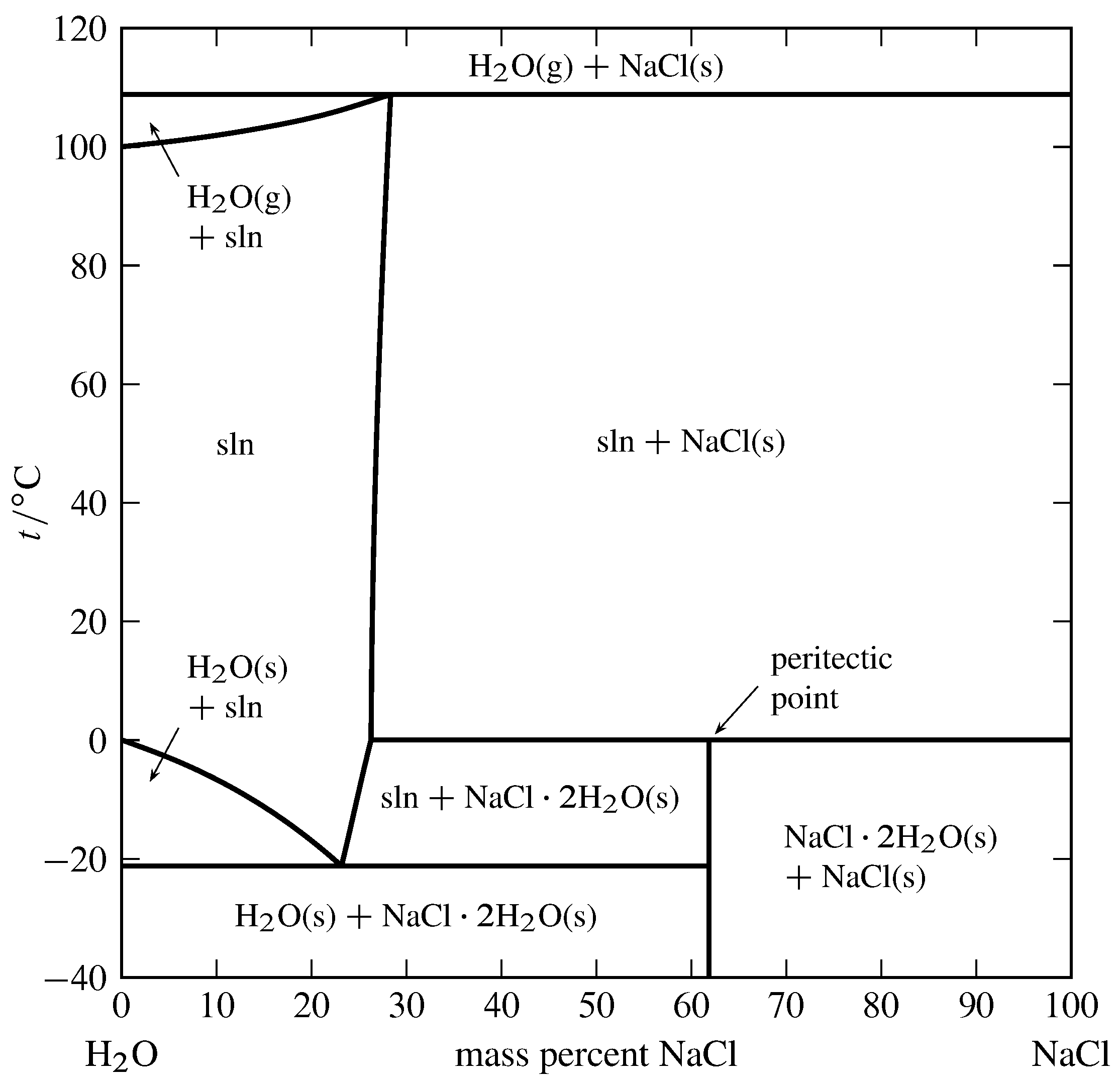
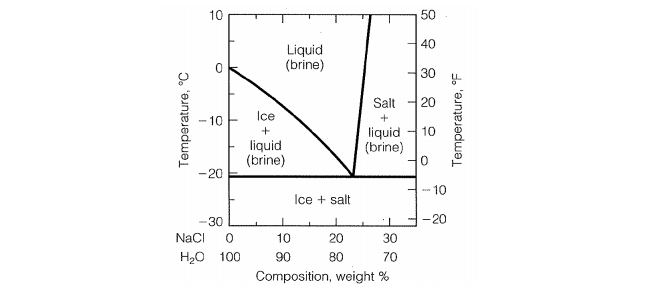
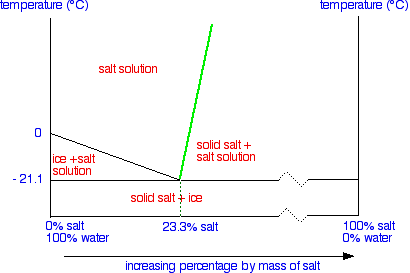
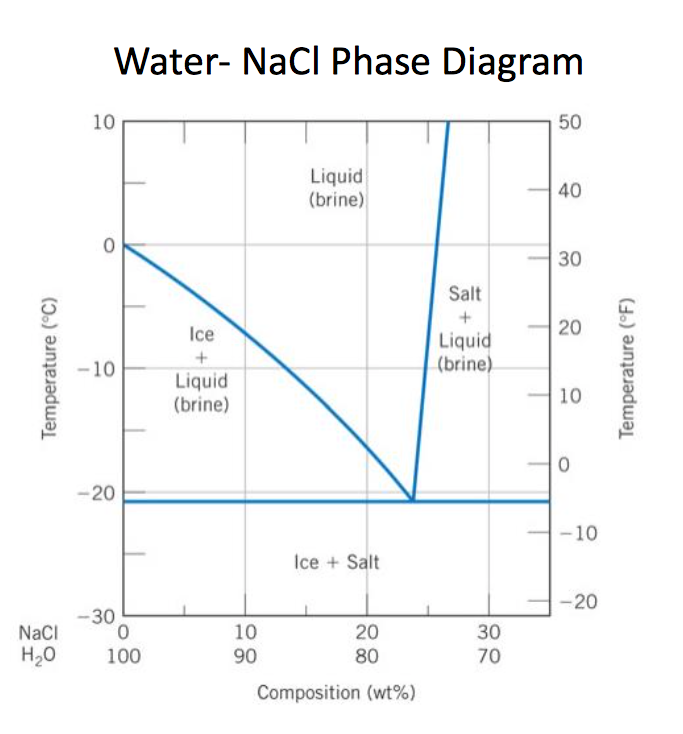
6. 2 Salt hydrates If the solid phase is an anhydrous salt the solubility dif ference depends only on isotopic effects in the solution. If the solid phase is a hydrate it will contain H^O if it is in equilibrium with ordinary water and D?0 if it is in equilibrium with heavy water. In the latter case it may be termed a deuterate. The Consider the salt - water phase diagram in Fig. 1. a) From the diagram, estimate the solubility limit of salt in water at + 20 degree C. b) At - 5 degree C and 30 weight % NaCl there are 2 phases present, brine and NaCI.2H20. Estimate the compositions of these phases.
Several phase diagrams for ternary salt solutions are shown in the tabs below. This includes Partial pressures in the carbon dioxide - ammonia - water system Solubility isotherms in the following systems: Sodium nitrate - sodium sulfate - water Sodium sulfate - magnesium sulfate - water Magnesium sulfate - potassium sulfate water
Salt water phase diagram
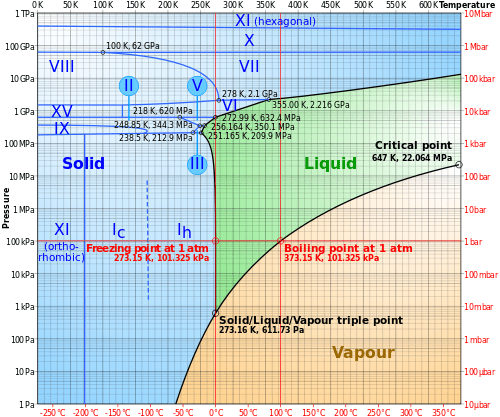
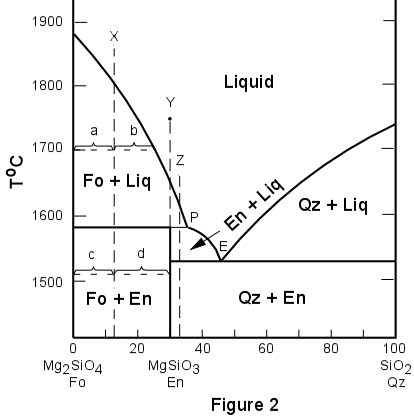
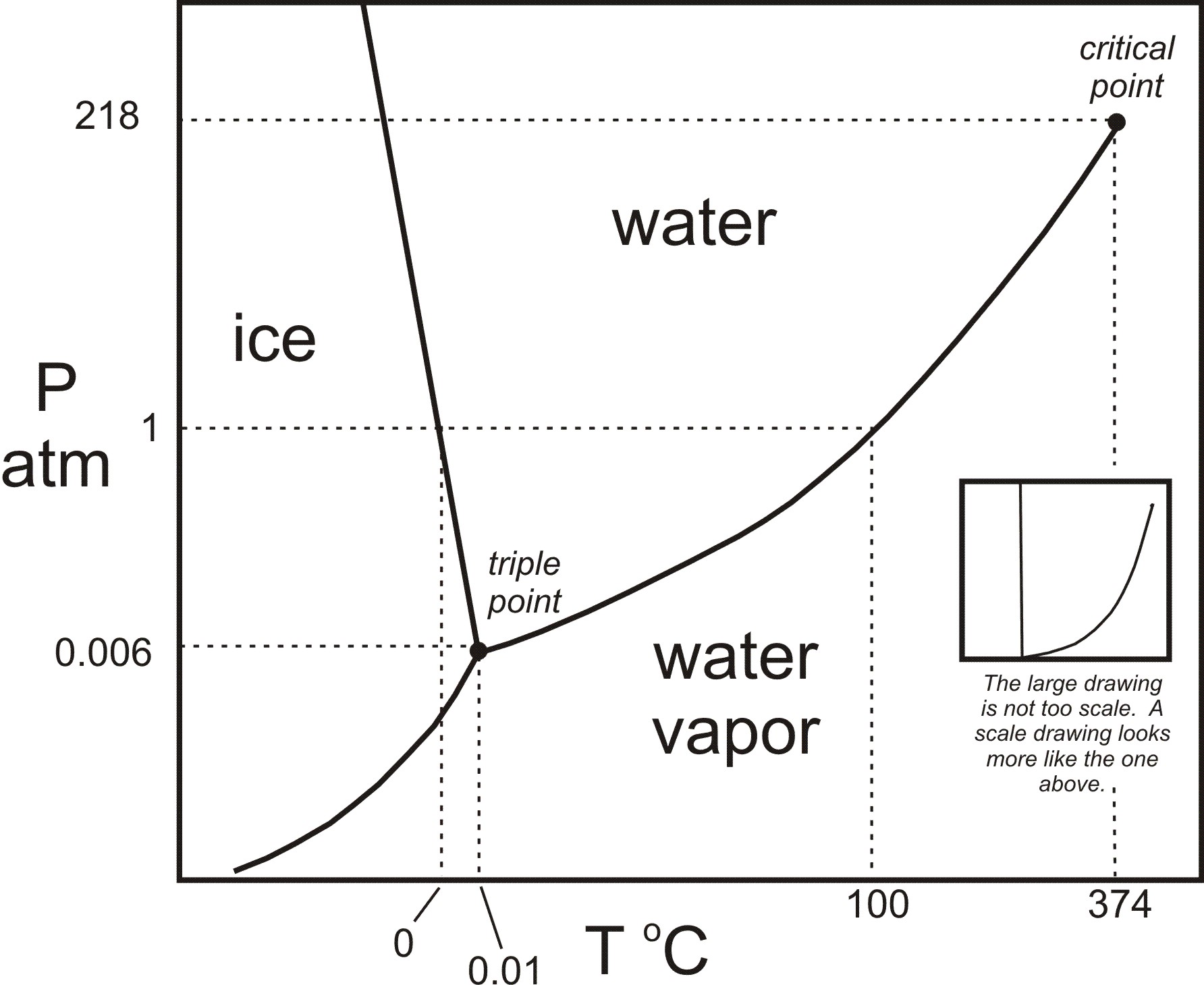
Phase diagram of water Note: for H2O melting point decreases with increasing pressure, for CO2 melting point increases with increasing pressure. WATER Covers ~ 70% of the earth’s surface Life on earth depends on water Water is a “universal” solvent Easily polluted; hard to purify. Phase Diagram Also called equilibrium or constitutional diagram Represent the relationships between temperature and composition and quantities of phase at equilibrium Pressure also influences phase structure Remains virtually constant in most applications Most phase diagrams at 1 atm Reading: West 11-12 Chem 253, UC, Berkeley Phases Sucrose/Water Phase Diagram Pure Sugar Temperature (°C) 0 20 40 60 80 100 Co =Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 40 60 80 100 Pure Water Adapted from Fig. 9.1, Callister 7e. Chapter 9 - 3 • Components :
Salt water phase diagram. temperatures and composition by constructing a phase diagram using temperature as the vertical axis and using the compositions H2O and NaCl to define a horizontal composition axis . At low temperatures, the "melt" in the H2O-NaCl system is salt water or "brine." Brines may be more or less salty depending on the ratio of NaCl to H2O, i.e ... Dissolving common salt (sodium chloride) in water unveils some fascinating aspects of this seemingly simple system. Salt-water phase diagram of the ternary system (NaCl + NaBO 2 + H 2 O) is significant in separate boron resources from brine. Solubilities and physicochemical properties bifurcated to density and refractive index for the ternary system at 288.15 and 308.15 K and 0.1 MPa were investigated experimentally by means of isothermal dissolution. COMPUTE WATER PHASE SALT CONTENT Water Phase Salt can be calculated by using either percent or grams of salt and moisture from the analysis. 100 % % % X Salt Moisture Salt WPS + = or X100 gSalt gMoisture gSalt WPS + = Example: Using the example above where a10 gram sample of smoked fish was found to have 60% moisture and 2.88% salt (0.288 g ...
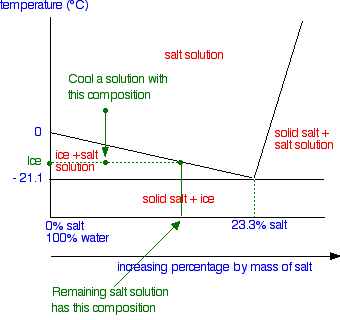
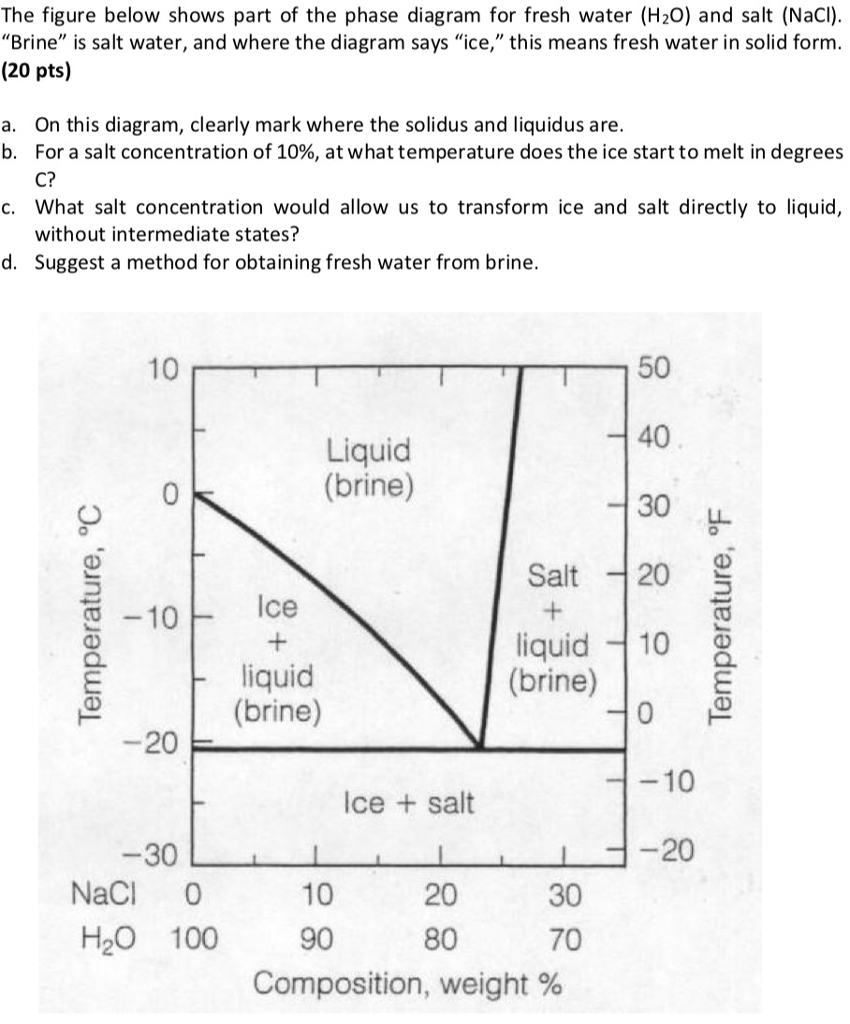
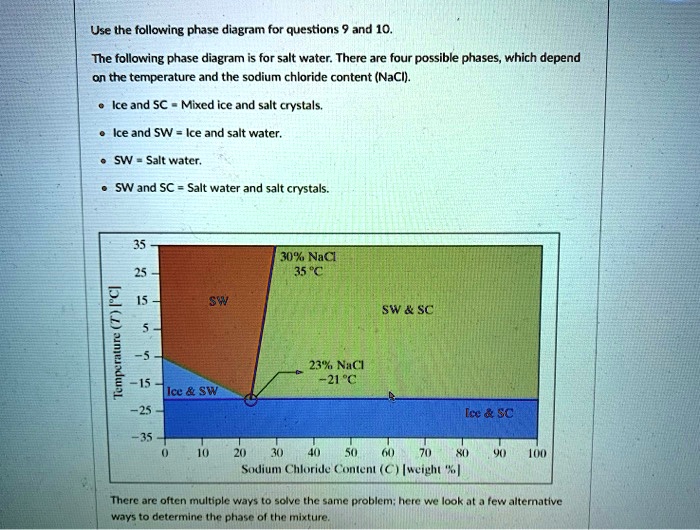
polymer-organic and salt-water phases. In the presented work, the phase diagrams of the water-polymer biphasic system PEG 6000-sodium citrate (C 6 H 5 O 7 Na 3)-water and the concentration effect of the sodium nitrate on the position of the binodal, on the value of the separating ability of the system were investigated. Phase diagram for the trisodium phosphate – water system. The phase diagram consists of six different branches, including ice. Experimental data are marked with red circles. the calculated equilibrium curve is marked with a black line. 05.02.2022 · The diagram below Shows How Salt is Removed from Sea Water – Model Answer 1. The flow chart gives information about the process of producing clean water from saltwater so that it can be used for drinking. Overall, seawater has to follow four main steps to be drinkable, beginning with the pretreatment filter stage and ending with the storage ... The labelled areas in the phase diagram. These areas all show what you would see if you had a particular mixture of salt and water at a given temperature. For example, if the temperature was below -21.1°C, you would always see a mixture of solid salt and ice. There would never be any liquid whatever proportions of salt and water you had.
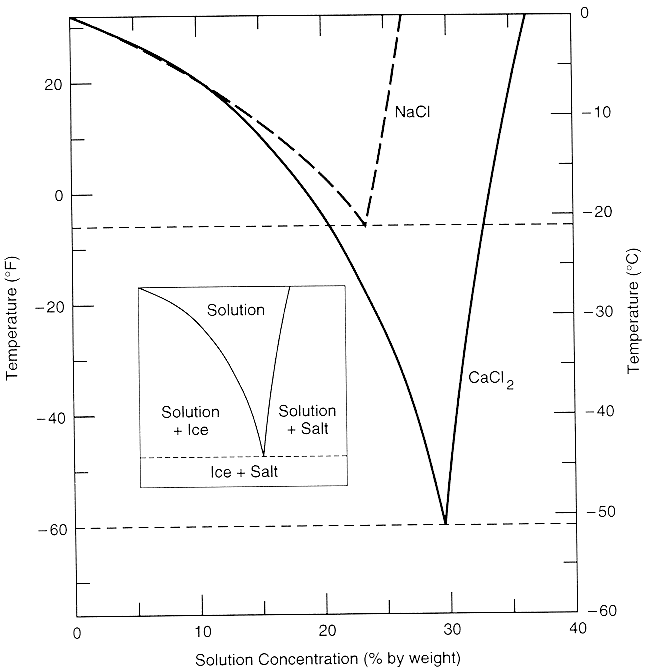
Aug 15, 2020 · The labeled areas in the phase diagram. These areas all show what you would see if you had a particular mixture of salt and water at a given temperature. For example, if the temperature was below -21.1°C, you would always see a mixture of solid salt and ice. There would never be any liquid whatever proportions of salt and water you had. Phase diagrams for salts having several hydrates can be even more complex, so another example will be given for the system Na +-SO 4 2--H 2 O [Steiger.etal:2008] Title: Crystallization of sodium sulfate phases in porous materials: The phase diagram Na2SO4-H2O and the generation of stress Author: Steiger, Michael; Asmussen, Sönke (Fig. 4). Phase diagrams of mixtures Mixtures of two salt hydrates were prepared on a mole ratio basis, ground lightly together, and samples of approximately 20 g were placed in an open test-tube and melted in an oil bath at ≤10 °C above the melting point. Transcribed image text: 2. This question pertains to the salt-water phase diagram shown below: Liquid (brine) Salt Ice Temperature (°C) Liquid (brine) Temperature (°F) Liquid (brine) Ice + Salt 20 NECE-300 1 0 HO 10090 20 30 Composition (wt%) (a) 100 g of salt is added to a beaker containing 750 g of water at 5°C and sufficiently mixed to dissolve all of the salt.
Phase Diagram for H2O Colligative Properties • Elevation of the normal boiling point • Lowering of the normal freezing point Elevation of the normal b.p. Super Slurper Super Slurper • "Slurper" molecules are polymers with hydrophilic ends that grab onto water molecules. • Sodium salt of poly(acrylic acid). •R-COO-, Na+ Osmosis/Osmotic Pressure
The phase diagram of salt (NaCl) and water. Source. Let us walk through two examples: When the concentration of salt is 5% and the temperature is -10°C we end up in the red area: ice + salt water. This means that there is both salt water and ice in this mixture.
Phase Diagram of Salt Water: Here is the phase diagram of salt water, i.e. water (H 2 O) with some dissolved salt (NaCl). Now let the mixture freeze or solidify. The fact that this happens at rather low temperatures compared to the freezing temperature of steel, for example, is completely irrelevant for the general considerations we are doing here.
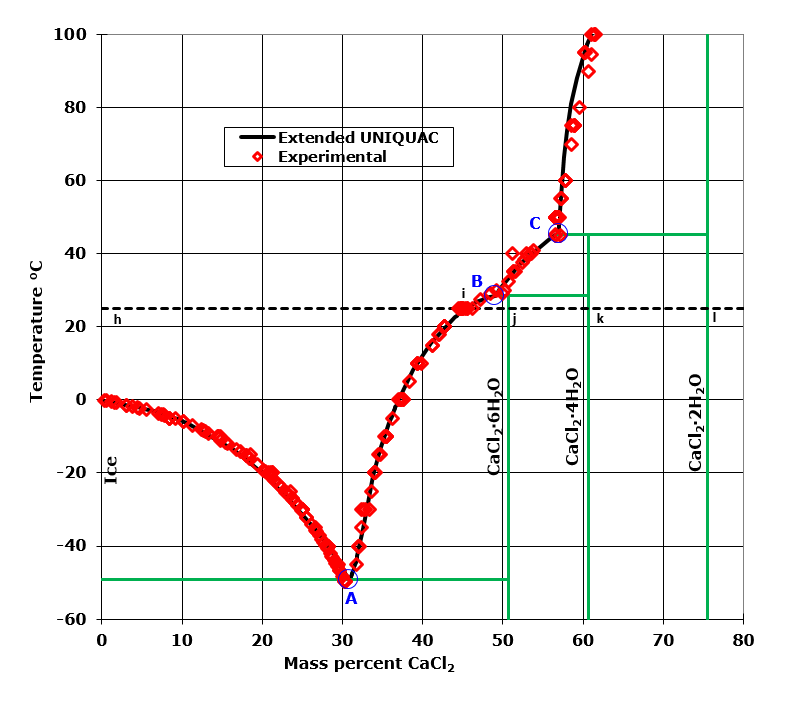
Phase diagram of the NaCl-H 2 O system and the principle of determining the solubility equilibrium curve by the TG method. A quasi-isothermal experiment is also possible. The sample is rapidly heated up to ca. 340 K and held at that temperature.
Solubility and phase diagram Water can only dissolve up to 26.4%wt of NaCl at 15 ºC, slightly increasing with temperature; see the phase diagram is presented in Fig. 1. The interest is just on liquid solutions, since the components do not mix in the solid state, and the amount of salt vapours can be neglected below say 1000 ºC.
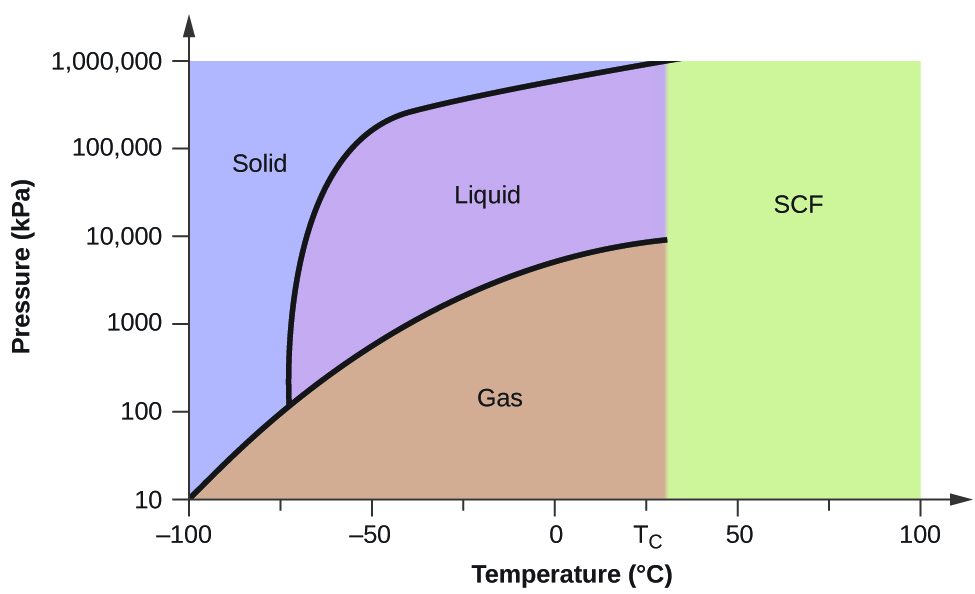
Determining the State of Water Using the phase diagram for water given in , determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 °C and 0.3 kPa (f) 50 °C and 0.3 kPa. Solution
Paperback. Pub Date: 2005 05 of Pages: 269 in Publisher: Tianjin University Press. salt-water system phase diagram and its application system about the principles of the phase diagram. draw two to five yuan salt-water system phase diagram phase diagram understanding of the calculation of the phase diagram and the phase diagram of the experimental method. application the Pizer electrolyte ...
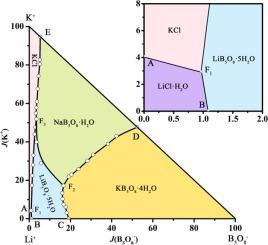
Phase diagrams for two quaternary salt solutions are shown in the tabs below. This type of phase diagrams are for example useful when designing fractional crystallization processes. The sodium chloride, potassium chloride, calcium chloride - water system.
the steps of the freezing-melting process used for the desalination process were reported in previous work (badawy, 2016): (a) freezing of seawater samples with a degree of crystallization of...
FTsalt - FACT Salt Phase Diagrams (351) Click on a system to display the phase diagram.
The stable-phase diagram of the quaternary system K +, Rb +, Cs + //SO 42- -H 2 O at T = 323.2 K consists of four invariant points, nine univariant curves, and six crystallization regions.
A typical phase diagram of a salt-water system shows that the EFC process can be represented by the paths A B E or C D E in Fig. 1. The salt concentration …
Saline water (more commonly known as salt water) is water that contains a high concentration of dissolved salts (mainly sodium chloride).The salt concentration is usually expressed in parts per thousand (permille, ‰) and parts per million (ppm). The United States Geological Survey classifies saline water in three salinity categories. Salt concentration in slightly saline water is around ...
Sucrose/Water Phase Diagram Pure Sugar Temperature (°C) 0 20 40 60 80 100 Co =Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 40 60 80 100 Pure Water Adapted from Fig. 9.1, Callister 7e. Chapter 9 - 3 • Components :
Phase Diagram Also called equilibrium or constitutional diagram Represent the relationships between temperature and composition and quantities of phase at equilibrium Pressure also influences phase structure Remains virtually constant in most applications Most phase diagrams at 1 atm Reading: West 11-12 Chem 253, UC, Berkeley Phases
Phase diagram of water Note: for H2O melting point decreases with increasing pressure, for CO2 melting point increases with increasing pressure. WATER Covers ~ 70% of the earth’s surface Life on earth depends on water Water is a “universal” solvent Easily polluted; hard to purify.









![PDF] Separating NaCl and AlCl3·6H2O Crystals from Acidic ...](https://d3i71xaburhd42.cloudfront.net/36471df76e1993c8a2064532a692c9bfaba2b3b1/3-Figure1-1.png)




0 Response to "36 salt water phase diagram"
Post a Comment