39 energy diagram transition state
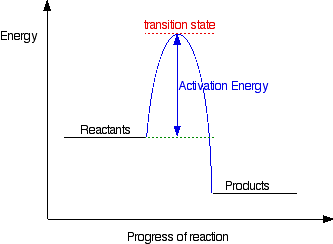
a transition state. KEYWORDS: Reaction, Energy , Rate, Cation INTRODUCTION The easiest way to understand the difference between a transition state and an intermediate is to use what is commonly called a reaction (energy) diagram, like the one below. methylpropane with iodide, we know that the rate carbocation. draw the transition state of a reaction. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ‘ reaction coordinate ’, tracing from left to right the progress of the reaction from starting compounds to final products.
In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are "captured ...

Energy diagram transition state
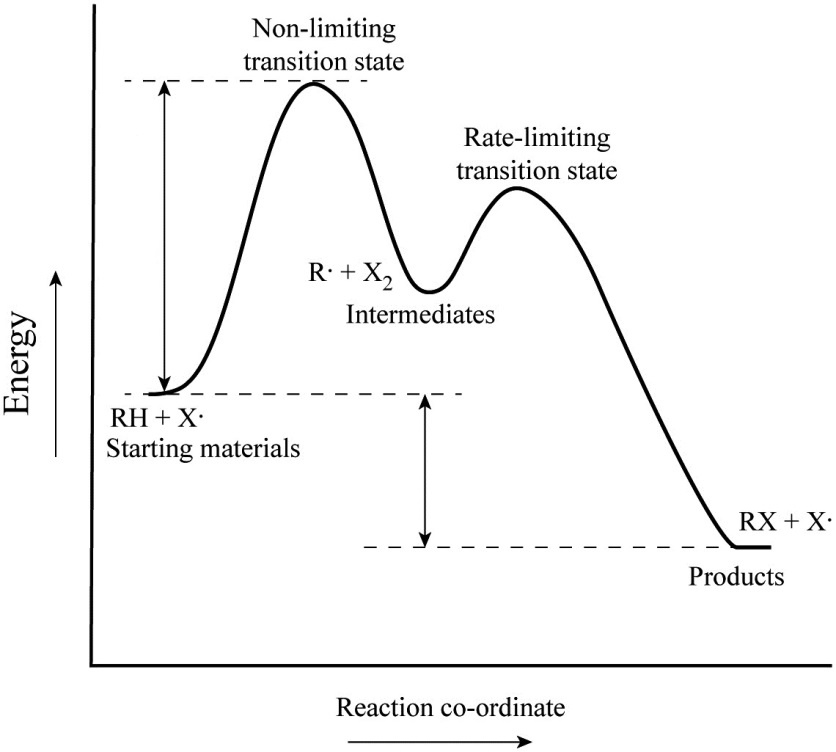
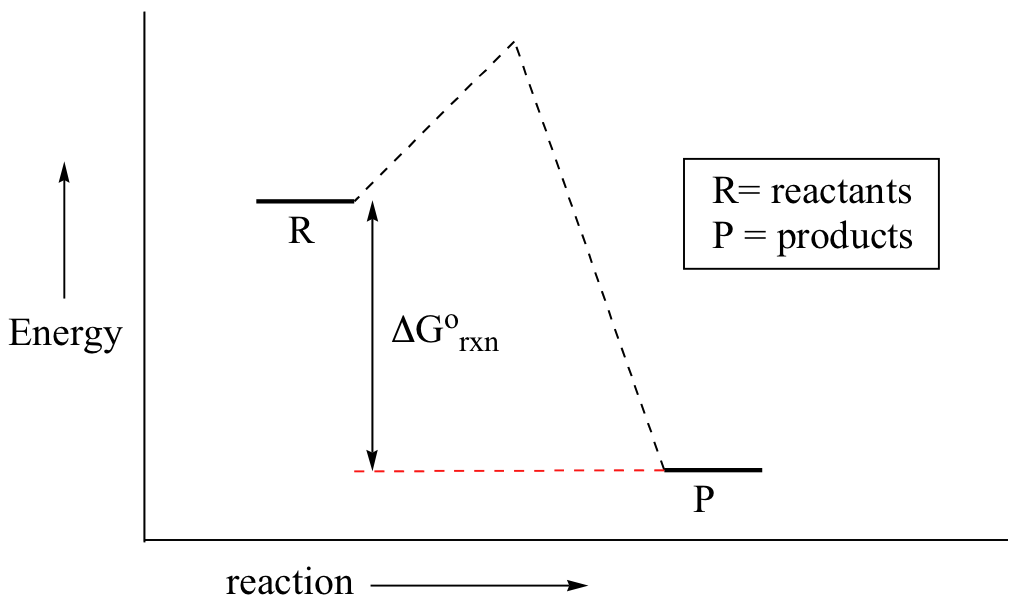
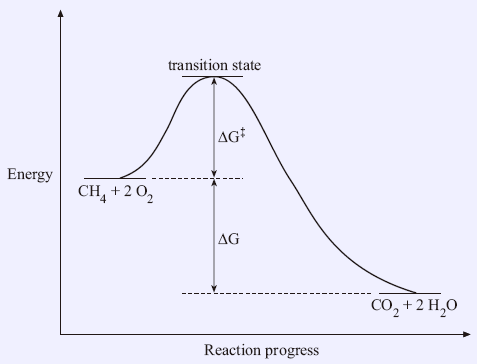
Potential energy Diagram of Reactions with Reactant, Product and Transition State The transition state of an organic reaction is a specific configuration along the reaction coordinate and corresponds to the highest potential energy along with this reaction coordinate. Furthermore, the transition state is not an actual molecule that can be isolated, and therefore it is often marked with the double dagger or star symbol to differentiate. Each step has its own reaction rate and transition state. The free-energy diagram is shown in Figure 1.3. For multistep reactions, the slowest step is called rate-determiningstep (or rate-limiting step ). The reaction rate of the overall reaction is equal to the rate of rate-determining step. The saddle point represents the highest energy point lying on the reaction coordinate connecting the reactant and product; this is known as the transition state. A reaction coordinate diagram may also have one or more transient intermediates which are shown by high energy wells connected via a transition state peak.
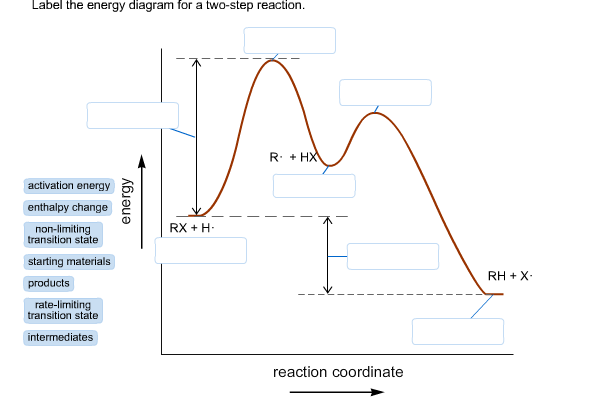
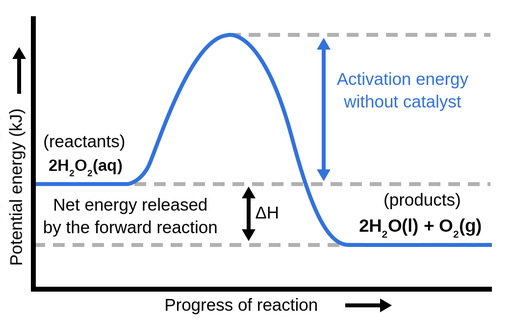
Energy diagram transition state. In this video, I go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. I'll ... Energy Energy Diagrams 6 • Transition state ‡: - An unstable species of maximum energy formed during the course of a reaction. - A maximum on an energy diagram. • Activation Energy, ∆G‡: The difference in Gibbs free energy between reactants and a transition state. - If ∆G‡ is large, few collisions occur with sufficient This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... The transition state is the point in the reaction with the highest energy level, and the difference in energy between the reagents and transition state is called the activation energy (often abbreviated as Ea). This Pin was discovered by Jessica L. Santos. Discover (and save!) your own Pins on Pinterest.The SN2 Reaction Energy Diagram ...
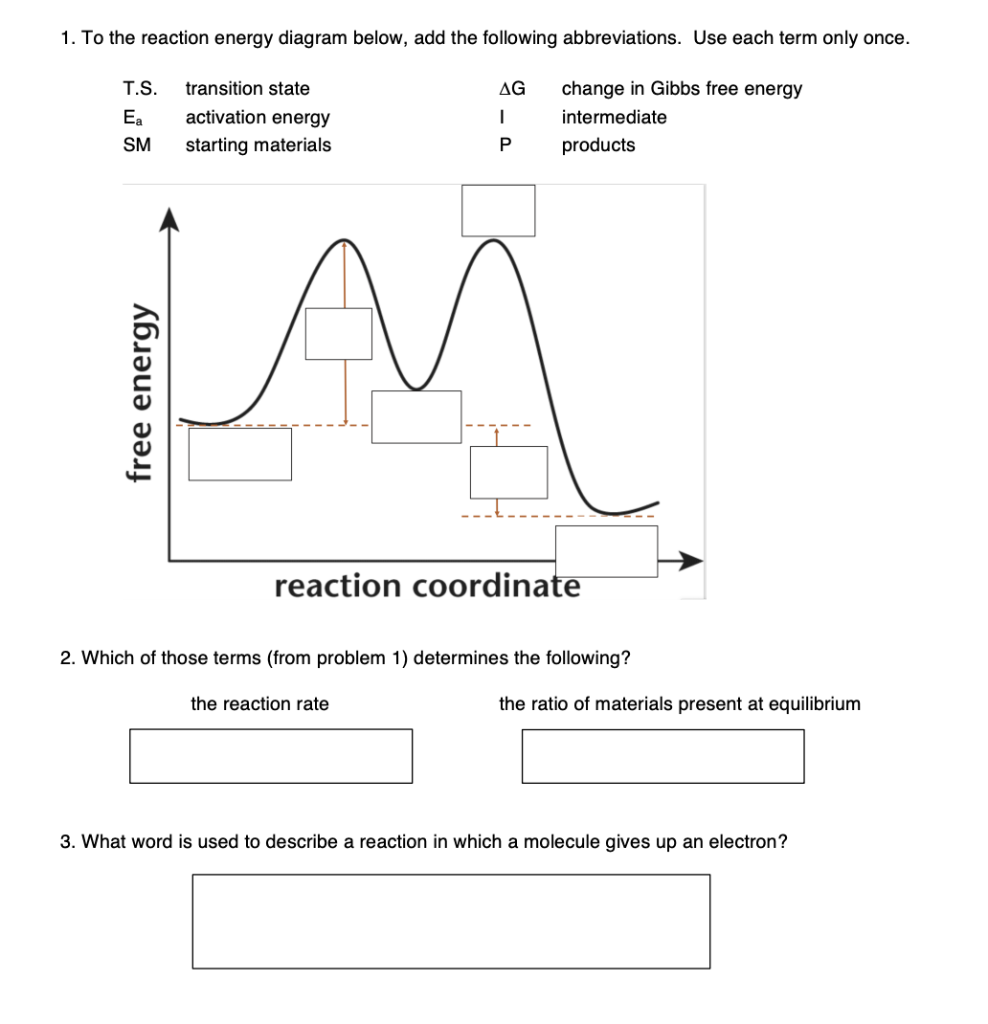
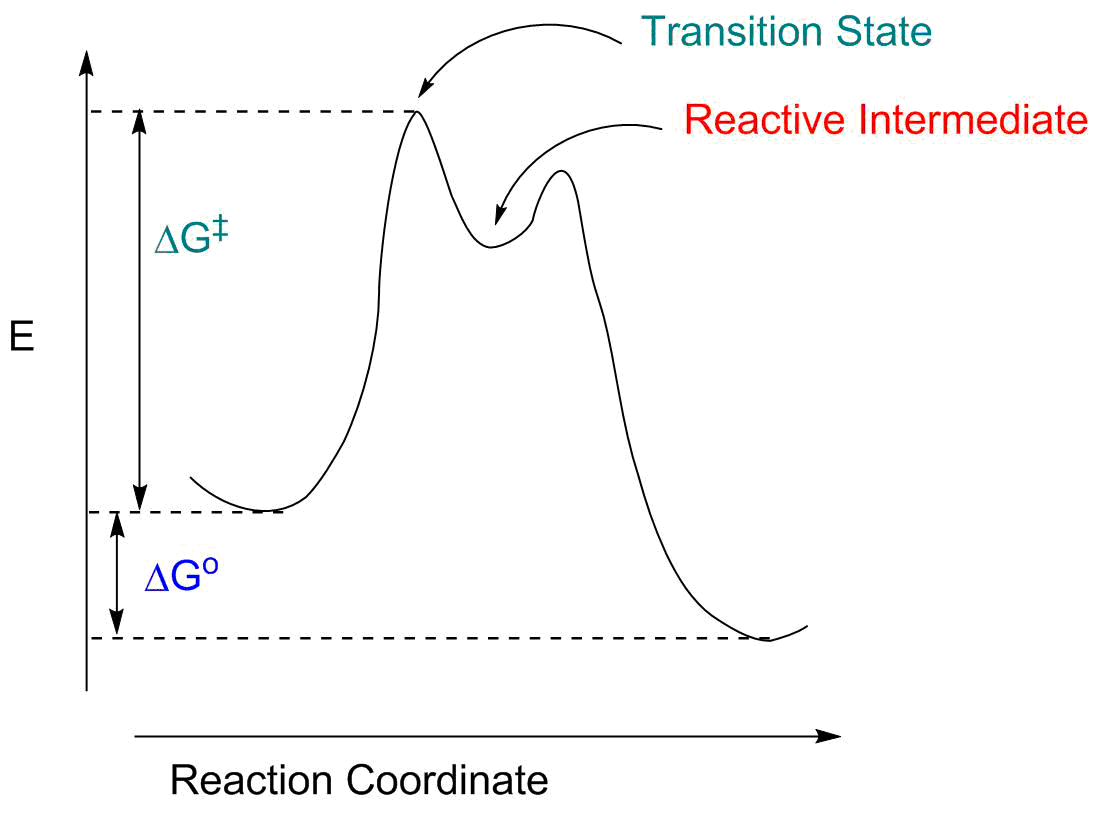
The transition state is the higher energy point between two minima along the reaction coordinate. Each step in a mechanism will have a transition state. The transition states are often very difficult to identify and during a reaction the molecules exist in this state for essentially zero time. ΔΔG‡ is the difference in the transition state energies for each process: ... during the 1980s, as methods of auxiliary mediated asymmetric synthesis advanced, "ee" spawned the term "de" (diastereomeric excess). The idea was that ... Energy diagram and reaction scheme for a kinetic resolution. Transition state is the highest point (or points) on the reaction coordinate diagram. Those are the "peaks" or the "hills" in the picture. A more strict definition is that a transition state is a molecular entity that has a lifetime no longer than a vibration that exhibits some structural characteristics of both the reactants and the products. between the reactant energy and the transition state at the peak of the diagram. The activation energy for the reverse reaction, E a(rev), is the difference between the product energy and transition state at the peak of the diagram. ΔH r is the difference between the potential energy of the reactant and the potential energy of the product. a.
1. Enantiomeric Transition State Structures 2. Diastereomeric Transition State Structures 3. Energy/Reaction Coordinate Diagrams 4. Felkin-Ahn Model for Cram's Rule E. Asymmetric Synthesis 1. Chiral Auxiliaries 2. Chiral Catalysts F. Measurement of Stereoselectivity 1. Diastereomeric Excess, de 2. Enantiomeric Excess, ee 3. Direct ... The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states. If the electron in the atom makes a transition from a particular state to a lower state, it is losing energy. Chapter 6—Nuclear Energy Levels 6-2 number, T, is an integer or half-integer that measures a property that results if neutron and proton coordinates were interchanged. Figure 6-1 shows these quantum numbers for each excited state in the notation J P, T.These quantum numbers are results of the basic This is part 3 of a four part series in the Energy Diagram Module. Stay tuned for Part 4! Click on the following links to see earlier parts: Part 1. Part 2. Sometimes reactions are more complex than simply a transition state (Graph 3), which would represent a single step in the reaction mechanism.
Sep 16, 2021 · At the very top of the energy barrier, the reaction is at its transition state (TS), which is the point at which the bonds are in the process of breaking and forming. The transition state is an ‘activated complex’: a transient and dynamic state that, unlike more stable species, does not have any definable lifetime. It may help to imagine a transition state as being analogous to the exact moment that a baseball is struck by a bat.
The peak of this energy diagram represents the transition state: an intermediate stage in the reaction from which the reaction can go in either direction. Reactions with a high activation energy will proceed very slowly, because only a few molecules will obtain enough energy to reach the transition state - even if they are highly exergonic.
Transition state theory (TST), also called activated complex theory, is often introduced in general chemistry courses when discussing kinetics. A reaction energy diagram is used to follow the progress of the reaction from reactants through a transition state to products (see figure 1). The reaction energy diagram plots the
Transition State in Energy Diagram Definition An energy diagram refers to the plot which shows the reactants' relative potential energies, its transition states, as well as the products progression of the reaction with time. It is a plot between potential energy and reaction progress or time.
Starting with the total energy of the system E= = n i e i, relative to the ground state, substitute for the number of molecules, n i, in energy state e I, using the Boltzmann distribution in the last equation of Step 3 and then sum to arrive at for non-interacting molecules. is the ground state energy.
Energy diagrams are used to represent the change in energy for the molecules involved in a chemical reaction. The free energy is measured along the y- axis, and the reaction coordinate is plotted on the x- axis. The reaction coordinate indicates the progress of the conversion of reactants to products. Peaks on the energy diagram represent the transition states, whereas the valleys represent the reactive intermediates.
That's the energy it takes for the reaction to occur. So it's from the reactants to the highest point on the curve in the transition state. So up here we have the transition state. And so the difference between the reactants and the transition state, as in 1, this difference, is what we call the activation energy. You might see that as E sub a.
The energy changes for the above reaction can be represented in the energy diagram shown in Fig. 7.1. S N 2 is a single-step reaction, so the diagram has only one curve. The products CH 3 OH and Br - are in lower energy than the reactants CH 3 Br and OH - , indicates that the overall reaction is exothermic and the products are more stable.
Both of the triplet state transitions are diagrammed on the right-hand side of the Jablonski energy profile illustrated in Figure 1. The low probability of intersystem crossing arises from the fact that molecules must first undergo spin conversion to produce unpaired electrons, an unfavorable process.
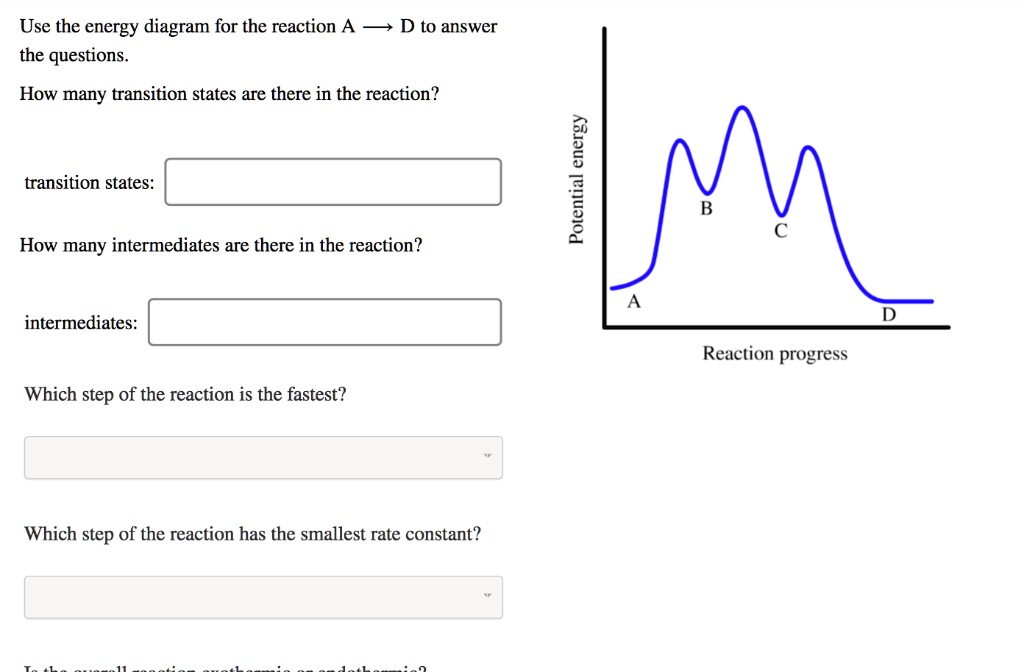
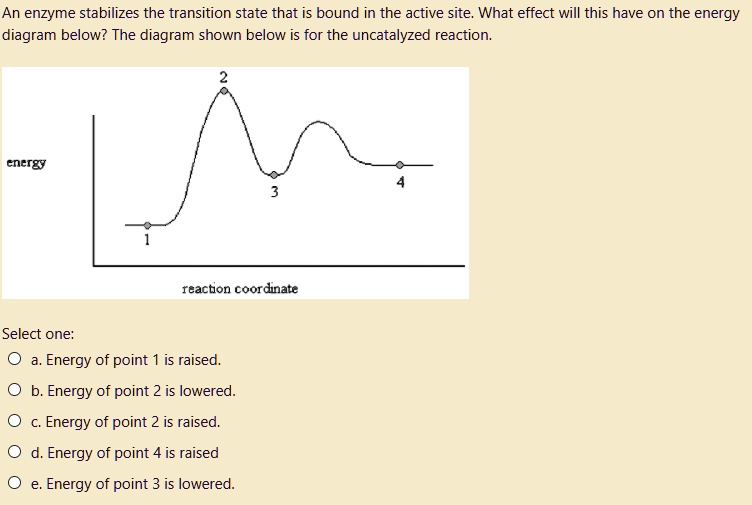
Best Answer. This is the best answer based on feedback and ratings. Transcribed image text: Which point on the following energy diagram represents the transition state of the rate-limiting step? . Energy Structure View Available Hint (s) O O O Energy Structure View Available Hint (s) ОА Ов Ос What is the correct order of stability for the ...
the beginning state. The energy of the TS≠ must be closer to the beginning state. There’s just no other way to draw the diagram. We call this TS≠ “early” since the structure of the transition state has not evolved far from its starting point. Case 2: the ending state is higher than the beginning state – In this case
the transition state is defined as the attained by the activated complex configuration along with the reaction coordinate where the potential energy of the reaction is highest as compared to the reactants and the final product formed at the compilation of the reaction whereas the activated complex is the unstable complex formed at the …
The saddle point represents the highest energy point lying on the reaction coordinate connecting the reactant and product; this is known as the transition state. A reaction coordinate diagram may also have one or more transient intermediates which are shown by high energy wells connected via a transition state peak.
Each step has its own reaction rate and transition state. The free-energy diagram is shown in Figure 1.3. For multistep reactions, the slowest step is called rate-determiningstep (or rate-limiting step ). The reaction rate of the overall reaction is equal to the rate of rate-determining step.
Potential energy Diagram of Reactions with Reactant, Product and Transition State The transition state of an organic reaction is a specific configuration along the reaction coordinate and corresponds to the highest potential energy along with this reaction coordinate. Furthermore, the transition state is not an actual molecule that can be isolated, and therefore it is often marked with the double dagger or star symbol to differentiate.


















0 Response to "39 energy diagram transition state"
Post a Comment