38 maximum solubility phase diagram
Solid Solubility - an overview | ScienceDirect Topics 2.6 Structural analysis of Mg-RE alloys containing Gd. The maximum solid solubility of Gd in Mg is 23.5 wt.%. When added to Mg, Gd forms a Mg 5 Gd phase with a high melting point of 548 °C. Figure 2.1024 shows the as-cast microstructure (a) and the solution-treated micro-structure (b) of the Mg-14Gd binary alloy. The Al-Si Phase Diagram - Cambridge University Press currently accepted diagram, Figure 1, is based on the study by Murray and McAlister [1] in 1984. The melting points of Al and Si are, respectively, 660.45 and 1414 C, while the eutectic reaction occurs at 12.6 wt. % Si and 577 1 C. The maximum solubility of Si in Al occurs at the eutectic temperature and is 1.65 wt. %.
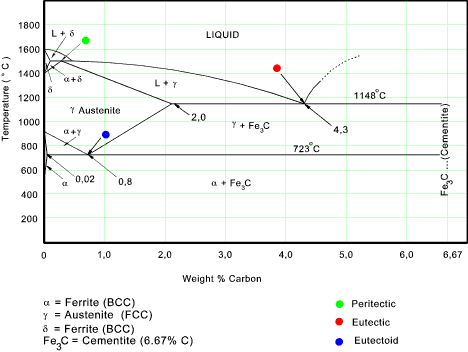
Iron-Carbon Diagram Explanation [PDF] - Mechanical E-Notes The maximum solubility of carbon is 0.1% at 1493 degrees centigrade. Fe(δ-Iron) possesses very low carbon content and thereby it possesses low hardness and that indicates it …

Maximum solubility phase diagram
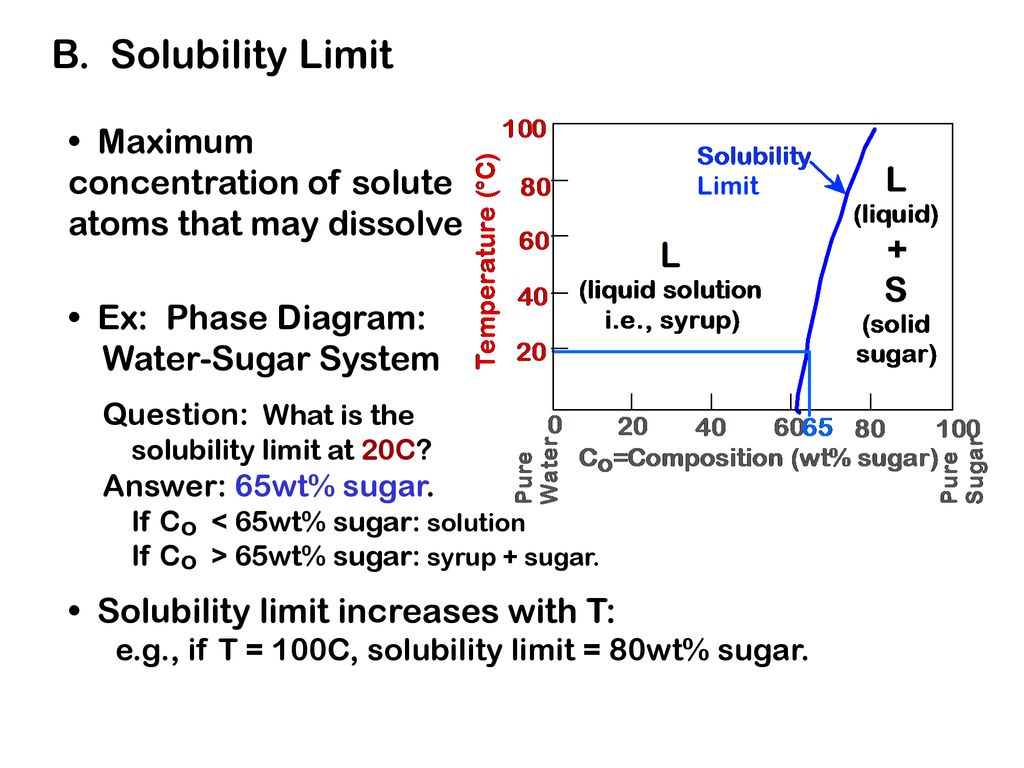
What is Alpha in phase diagram? - JanetPanic.com Solubility Limit of a component in a phase is the maximum amount of the component that can be dissolved in it (e.g. alcohol has unlimited solubility in water, sugar has a limited solubility, oil is insoluble). What is the binary Al-Si phase diagram? The binary Al-Si phase diagram was initially studied by Fraenkel of Germany in 1908. Solved i. 11. Answer the following questions based on the ... What is the maximum solubility of Pb in Mg at 400 °C? b. What is the composition of the intermetallic compound? c. What is the composition of the intermetallic compound? d. For a 90 st% Pb alloy, what phases are present at the following temperatures: i. T = 600; Question: i. 11. Answer the following questions based on the Mg-Pb phase diagram ... Phase equilibria: solubility limit Phase equilibria: solubility limit Introduction –Solutions – solid solutions, single phase –Mixtures – more than one phase • Solubility Limit: Max concentration for which only a single phase solution occurs. Sucrose/Water Phase Diagram Sugar) 0 20 40 60 80 100 C o=Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S
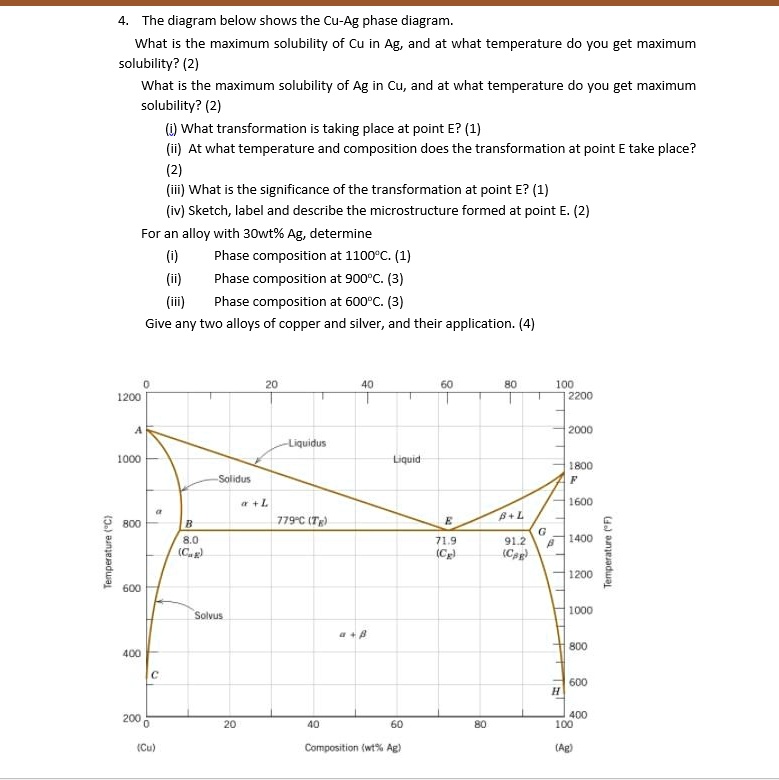
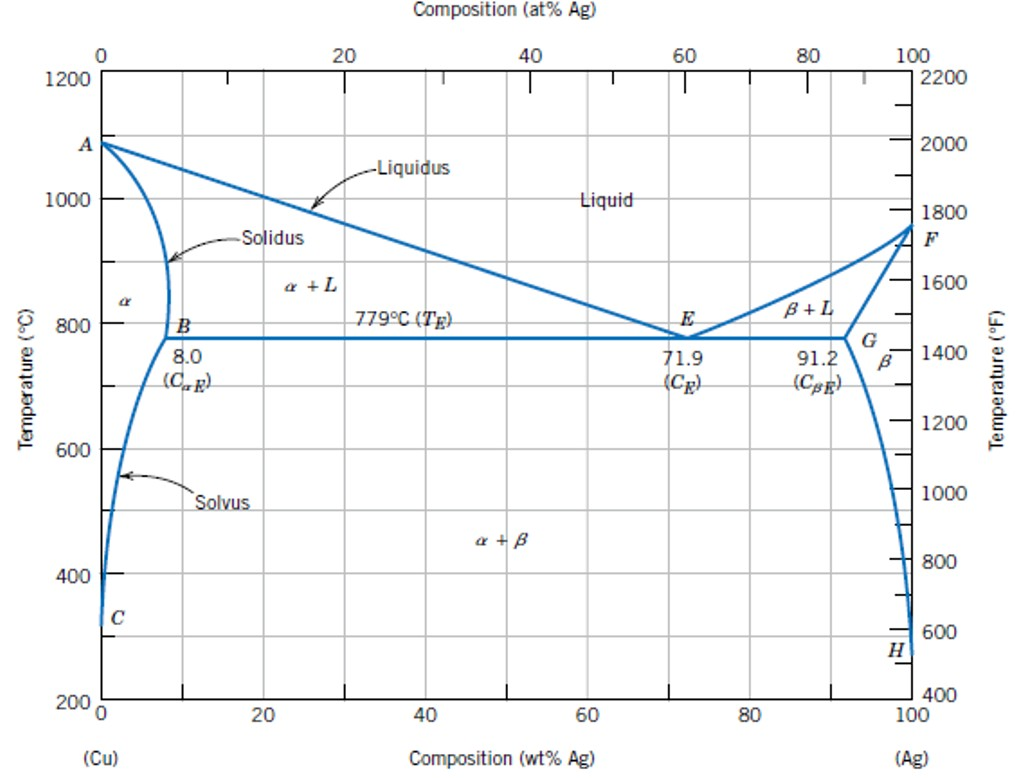
Maximum solubility phase diagram. What is the maximum solubility of Cu in Ag and at what ... Moreover, what is solubility limit in phase diagram? Solvent - host or major component in solution, solute - minor component (Chapter 4). Solubility Limit of a component in a phase is the maximum amount of the component that can be dissolved in it (e.g. alcohol has unlimited solubility in water, sugar has a limited solubility, oil is insoluble). Shape Memory Alloy - an overview | ScienceDirect Topics The different phase transformations that characterize the shape memory effect can also be outlined by referring to the phase diagram of the material (see Fig. 9.3).In the initial undeformed and unloaded condition, the material is considered to be twinned martensite (bottom-left part of the diagram) as long as the temperature of the material is below the temperature A s as … PDF Phase Diagram - Surendranath College Phase Diagram 3 temperature will be noted. Then the temperature vs. Weight percentage of phenol will be plotted. From the graph, obtained maximum solubility temp and its corresponding composition; those L05 - Phase Diagrams.pdf - Materials Science, MSEN 222 ... Department of Materials Science and Engineering 3 Phase Equilibria: Solubility Limit • Solution - solid, liquid, or gas solutions, single phase • Mixture - more than one phase • Solubility Limit: Maximum concentration for which only a single-phase solution exists.
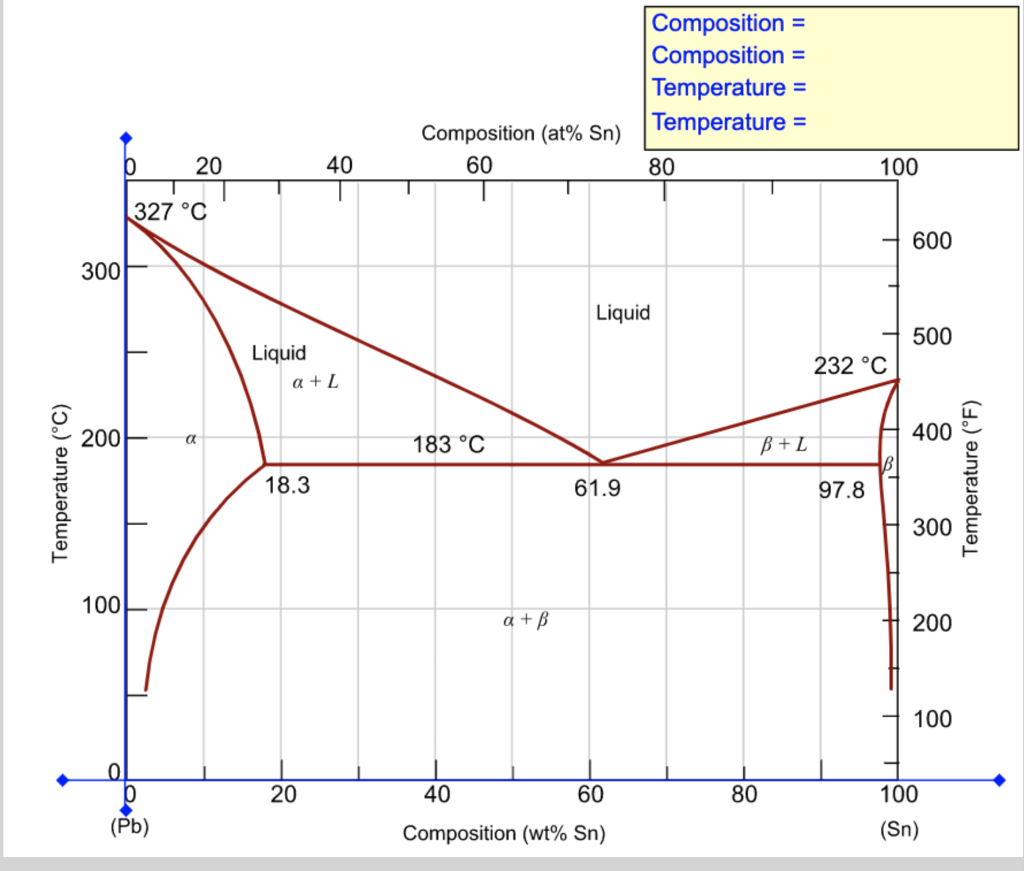
Chapter Outline: Phase Diagrams MSE 2090: Introduction to Materials Science Chapter 9, Phase Diagrams 3 Solvent - host or major component in solution,solute- minor component. Solubility Limitof a component in a phase is the maximum amount of the component that can be dissolved PDF Mat Sci Homework 6 SOLUTIONS SP2015 - Olin Use the following silver-copper phase diagram for Problems 5-9. 5. What are the solubility limits of Ag in (Cu) and Cu in (Ag)? Recall that (Cu) and (Ag) are the same as α and β, respectively. The solubility limit of Ag in (Cu) is 7.9 wt. % Ag. The solubility limit of Cu in (Ag) is 8.8 wt.% Cu. Note that these Solubility - Wikipedia Solubility (metastable, at concentrations approaching saturation) also depends on the physical size of the crystal or droplet of solute (or, strictly speaking, on the specific surface area or molar surface area of the solute). For quantification, see the equation in the article on solubility equilibrium.For highly defective crystals, solubility may increase with the increasing degree of … From the copper silver phase diagram Figure 107 the ... (b) From this same figure, the maximum solubility of Ag in Cu corresponds to the position of the -( + ) phase boundary at this temperature, or about 5 wt% Ag. Concept Check 10.5 Question: Below is a portion of the H 2 O-NaCl phase diagram: (a) Using this diagram, briefly explain how spreading salt on ice that is at a temperature below 0 C (32 F ...
Alloys - limited solubility of components in solid state ... How to read the phase diagram. ... The solvus line shows that the host substance A has its maximum solubility at a temperature of 700 °C and can store 20 % B atoms. At this temperature, the entire B atoms of the alloying element remain dissolved in the lattice structure of the host material A. Fe-C diagram - SlideShare 18/11/2013 · Iron–Carbon Phase Diagram • In their simplest form, steels are alloys of Iron (Fe) and Carbon (C). • The Fe-C phase diagram is a fairly complex one, but we will only consider the steel and cast iron part of the diagram, up to 6.67% Carbon. 3. Fe – C Equilibrium Diagram 4. ©2003 Brooks/Cole, a division of Thomson Learning, Inc. Thomson Learning ™ is a trademark … Phase decomposition and strengthening in HfNbTaTiZr high ... 15/02/2022 · According to the phase diagram of the (NbTa) x (TiZrHf) 1-x pseudo-binary alloy, the equiatomic HfNbTaTiZr HEA undergoes spinodal decomposition below temperature of 1298 K. However, the SEM observation by Senkov et al. showed that the size of the decomposed particles in HfNbTaTiZr are respectively 0.18, 0.28, 0.50 and 0.72 μm when aging for 4, 16, 64, and 256 … Eutectic system - Wikipedia On a phase diagram, the eutectic temperature is seen as the eutectic point (see plot on the right). Non ... Ethanol–water has an unusually biased eutectic point, i.e. it is close to pure ethanol, which sets the maximum proof obtainable by fractional freezing. "Solar salt", 60% NaNO 3 and 40% KNO 3, forms a eutectic molten salt mixture which is used for thermal energy storage in …
PDF The Al-Si Phase Diagram - Buehler, an ITW Company The currently accepted diagram, Figure 1, is based on the study by Murray and McAlister1 in 1984. The melting points of Al and Si are, respectively, 660.45 and 1414 °C, while the eutectic reaction occurs at 12.6 wt. % Si and 577 ± 1 °C2. The maximum solubility of Si in Al occurs at the eutectic temperature and is 1.65 wt. %.
THE SOLUBILITY LIMIT Solubility Limit: Max concentration for. which only a solution. occurs. • Ex: Phase Diagram: Water-Sugar System. Question: What is the. solubility limit at 20C? Answer: 65wt% sugar. If C. o < 65wt% sugar: sugar. If C. o > 65wt% sugar: syrup + sugar. • Solubility limit increases with T: e.g., if T = 100C, solubility limit = 80wt% sugar. Pure Sugar. Temperature (°C)
Iron-Carbon Phase Diagram Explained [with Graphs] - Fractory This phase is a solid solution of carbon in FCC Fe with a maximum solubility of 2.14% C. On further heating, it converts into BCC δ-ferrite at 1395°C. γ-austenite is unstable at temperatures below eutectic temperature (727°C) unless cooled rapidly. This phase is non-magnetic. δ-ferrite
Phase Diagrams Phase Diagrams. define and give examples of the following terms as applied to phase equilibria: component, system (two meanings),solubility limit, phase, microstructure, equilibrium phase diagram. sketch a binary (two-component) isomorphous (100% solid solution) phase diagram and label the regions of the diagram.
Solubility Limit - University of Portland Solution (a) From Figure 9.7, the maximum solubility of Cu in Ag at 500 °C corresponds to the position of the β-(α + β) phase boundary at this temperature, or to about 2 wt% Cu. (b) From this same figure, the maximum solubility of Ag in Cu corresponds to the position of the α-(α + β) phase boundary at this temperature, or about 1.5 wt% Ag.
PDF Chapter 9: Phase Diagrams - Florida International University Chapter 9: Phase Diagrams Phase A Phase B Nickel atom Copper atom. Chapter 9 - 2 Phase Equilibria: Solubility Limit Introduction - Solutions - solid solutions, single phase - Mixtures - more than one phase • Solubility Limit : Max concentration for which only a single phase solution occurs. Question: What is the solubility limit at 20°C?
PDF Phase diagrams - weizmann.ac.il The max solubility limit is always at the eutectic temperature. Example - Gibbs phase rule in eutectic diagram In the double phase regions: N = 1 (the diagram is plotted for fixed pressure, 1 atm) C = 2 P = 2 F = N + C -P F = 1
Solid Solution of Metals: With Diagram | Metallurgy If the mutual solid solubility is restricted (as in Cu-Ag system) to only those portions of the phase diagram that are linked to the pure elements, the solid solutions formed are called as primary (or terminal) solid solutions, which have same crystal structure as of solvent metals. If other phases are present in the system, they are usually called intermediate phases, or intermetallic phases ...
Phase diagrams - Weizmann Institute of Science Full Solubility (Cigar shaped diagram) In Cigar shape diagram there are three different regions: 1. Liquid (single phase) 2. Liquid + solid (double phase) 3. Solid solution (single phase) •Liquidus- the boundary line between the liquid region and the double phase region. •Solidus- the boundary line between the solid solution region and the double phase
IRON CARBON PHASE DIAGRAM - Higher Technological Institute Maximum solubility is 2.0 % C at 1130°C. ... Simplified Iron-Carbon phase diagram austenite ↔ pearlite (mixture of ferrite & cementite) Eutectoid reaction Redistribution of carbon by diffusion Austenite –0.76 wt% C Ferrite - 0.022 wt% C Cementite - 6.70 wt% C Pearlite y↔α + Fe 3 C. The Austenite to ferrite / cementite transformation in relation to Fe-C diagram. The Austenite to …
Iron Phase Diagram - Roy Mech The phased diagram includes four solid phases α Ferrite ..The solid solution of carbon in iron. At 0% C this is pure iron. BCC crystal structure. The maximum solubility of carbon in iron is 0,02% at 723oC. The carbon atoms are located in the crystal interstices. Austenite The solid solution of carbon in γ iron is called austenite .
Phase Diagrams – Chemistry - University of Hawaiʻi A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures (melting points, sublimation points, …
The Ni-Al phase diagram [15] and the solubility limits ... The Ni-Al phase diagram [15] and the solubility limits (solid lines) used in our model. Source publication Modeling heterogeneous interaction during SHS in the NiAl system: A phase-formation ...
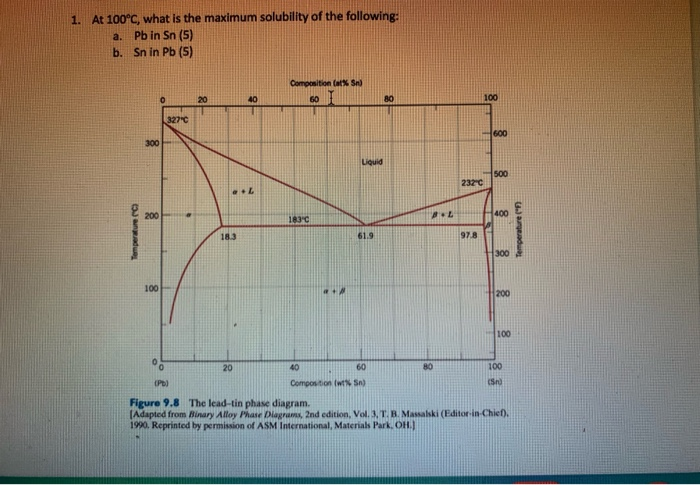
Solved At 100 C, what is the maximum solubility (a) of Pb ... At 100 C, what is the maximum solubility (a) of Pb in Sn? (b) of Sn in Pb? The lead-tin phase diagram is shown below. Round to the nearest whole number. Question: At 100 C, what is the maximum solubility (a) of Pb in Sn? (b) of Sn in Pb? The lead-tin phase diagram is shown below. Round to the nearest whole number.
Quiz - how to build a phase diagram - Southampton Quiz - how to build a phase diagram: question 2 of 4: Q2: the Alpha phase is: solid A. a mixture of solid A and liquid A. a little A dissolved into a solid that is mostly B ... what is the maximum solubility in the alpha solid solution? 50wt%A - 50wt%B. 90wt%A - 10wt%B. 10wt%A - 90wt%B ...
PDF Iron-Carbon Phase Diagram (a review) see Callister Chapter 9 Phases in Fe-Fe3C Phase Diagram ¾α-ferrite - solid solution of C in BCC Fe • Stable form of iron at room temperature. • The maximum solubility of C is 0.022 wt% • Transforms to FCC γ-austenite at 912 °C ¾γ-austenite - solid solution of C in FCC Fe • The maximum solubility of C is 2.14 wt %. • Transforms to BCC δ-ferrite at ...
gas-liquid chromatography - chemguide High solubility in the liquid phase means a high retention time. the temperature of the column. A higher temperature will tend to excite molecules into the gas phase - either because they evaporate more readily, or because they are so energetic that the attractions of the liquid no longer hold them. A high column temperature shortens retention ...
Phase diagram, solubility limit and hydrodynamic ... Cellulose solubility phase diagram (shaded areas) in EmimAc-DMSO (line corresponds to 2.5 EmimAc molecules bound to 1 AGU) and in EmimAc-water (line corresponds to experimental results) with dashed line being a possible continuation of C cell max in EmimAc-water. Concentrations are in wt%.
Phase Diagram: Meaning and Types | Material Engineering Hardness of pure material is very poor and maximum strength will be at the point of maximum solid solubility. Influence of Alloying Elements on Phase Diagram: Alloys elements addition always reduces eutectoid composition i.e.% of C while they may increase or decrease the eutectoid temperature.
Solubility Limits and Determining Phase Present ... For 60+ videos on Engineering Materials
Phase equilibria: solubility limit Phase equilibria: solubility limit Introduction –Solutions – solid solutions, single phase –Mixtures – more than one phase • Solubility Limit: Max concentration for which only a single phase solution occurs. Sucrose/Water Phase Diagram Sugar) 0 20 40 60 80 100 C o=Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S
Solved i. 11. Answer the following questions based on the ... What is the maximum solubility of Pb in Mg at 400 °C? b. What is the composition of the intermetallic compound? c. What is the composition of the intermetallic compound? d. For a 90 st% Pb alloy, what phases are present at the following temperatures: i. T = 600; Question: i. 11. Answer the following questions based on the Mg-Pb phase diagram ...
What is Alpha in phase diagram? - JanetPanic.com Solubility Limit of a component in a phase is the maximum amount of the component that can be dissolved in it (e.g. alcohol has unlimited solubility in water, sugar has a limited solubility, oil is insoluble). What is the binary Al-Si phase diagram? The binary Al-Si phase diagram was initially studied by Fraenkel of Germany in 1908.














0 Response to "38 maximum solubility phase diagram"
Post a Comment