39 molecular orbital diagram for h2- and bond order
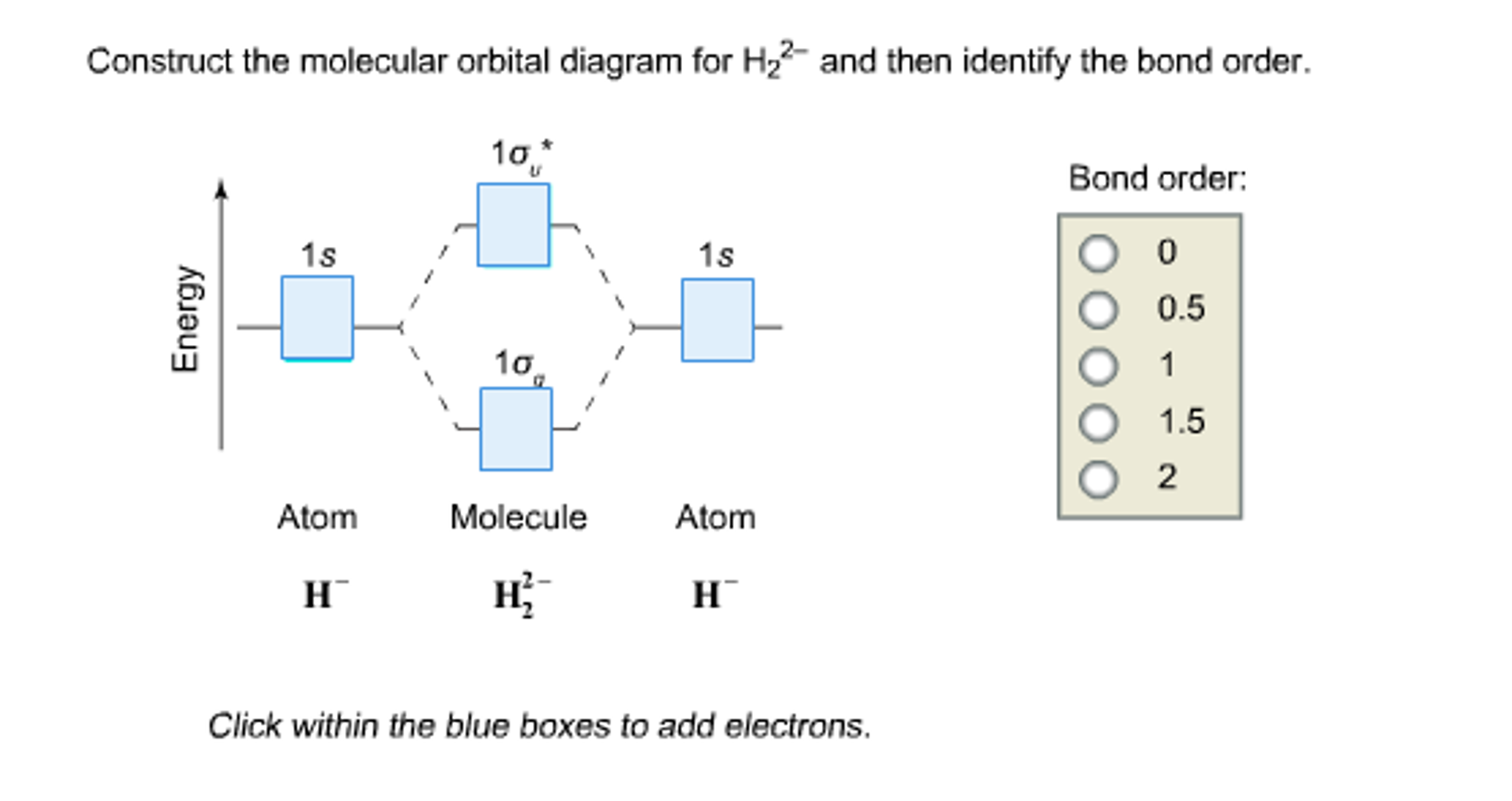
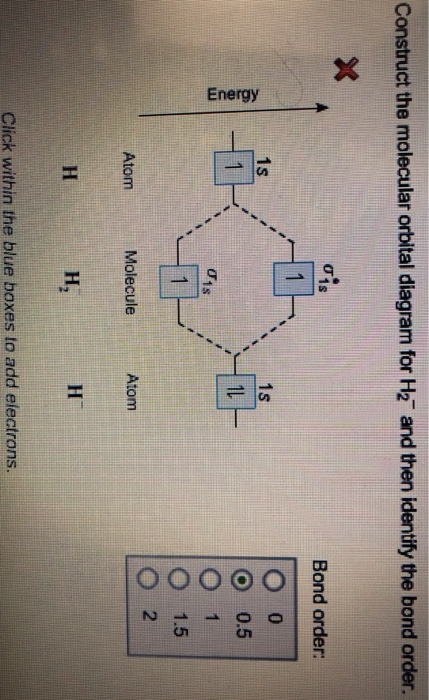
Construct The Molecular Orbital Diagram For H2 And Then ... Construct the molecular orbit diagram for H2- and then identify the bond order. Shortcut order: Click slim the blue box to include electrons. The concept used to settle this problem is based on molecular orbit diagram. You are watching: Construct the molecular orbital diagram for h2 and then identify the bond order OneClass: Construct the molecular orbital diagram for H2 ... 1 Nov 2019 Construct the molecular orbital diagram for H2 and then identify the bond order. Make sure you add electrons to the boxes corresponding to the MOs for the molecule and to the boxes corresponding to the AOs for the two atomic species. Bond order: 1s o 0.5 O 1.5 2 Atom Molecule Atom Click within the blue boxes to add electrons. Answer + 20
Construct The Molecular Orbital Diagram For H2 Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here.

Molecular orbital diagram for h2- and bond order
Solved Construct the molecular orbital diagram for H2- and ... Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Question: Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in . Is H2 a viable molecule for the molecular orbital ... - Quora Answer (1 of 2): According to the molecular orbital theory, a molecular is viable of its bond order is more than or equal to one. The bond order is defined as number of bond between to two atoms of that molecule. It is calculated as the difference of electrons in bonding molecules and anti-bondin...
Molecular orbital diagram for h2- and bond order. Draw a molecular orbital diagram of N2 or O2 with magnetic ... Hint: Generally the molecular orbital diagrams are used to understand the bonding of a diatomic molecule. You should know that molecular orbital diagrams are used to deduce magnetic properties of a molecule; they also help us to find out the bond order of the molecule. Draw the molecular orbital energy diagram for oxygen ... Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown: (i) Electronic configuration: (ii) Bond order: Here N b = 8; N a = 4 The two oxygen atoms in a molecule of oxygen are united through two covalent ... PDF Molecular Orbital (MO) Theory of the H2 molecule Testin g qualitative MO theory prediction of Bond Order with experiment for homonuclear diatomics made from elements in the 1st row of the Periodic Table (using the "Molecular Orbital Aufbau" principle): BondOrder [# ' # ' ]/2≡−bondinge s antibondinge s [D.A. McQuarrie, Quantum Chemistry] Molecular Orbital Theory Concept & Diagrams | What is ... The bond order is calculated by adding up the electrons in bonding molecular orbitals, subtracting the anti-bonding orbitals, and dividing by two (because two electrons occupy each orbital).
chemical bonding - Molecular orbitals of H2 and He2 ... The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure 13. On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding ... Molecular Orbital (MO) Diagram of H2 - YouTube Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ... Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... How to Make the Molecular Orbital Diagram for H2- (Bond ... This video discusses how to draw the molecular orbital (MO) diagram for the H2- ion. The bond order of H2- is also calculated and the meaning of this number ...
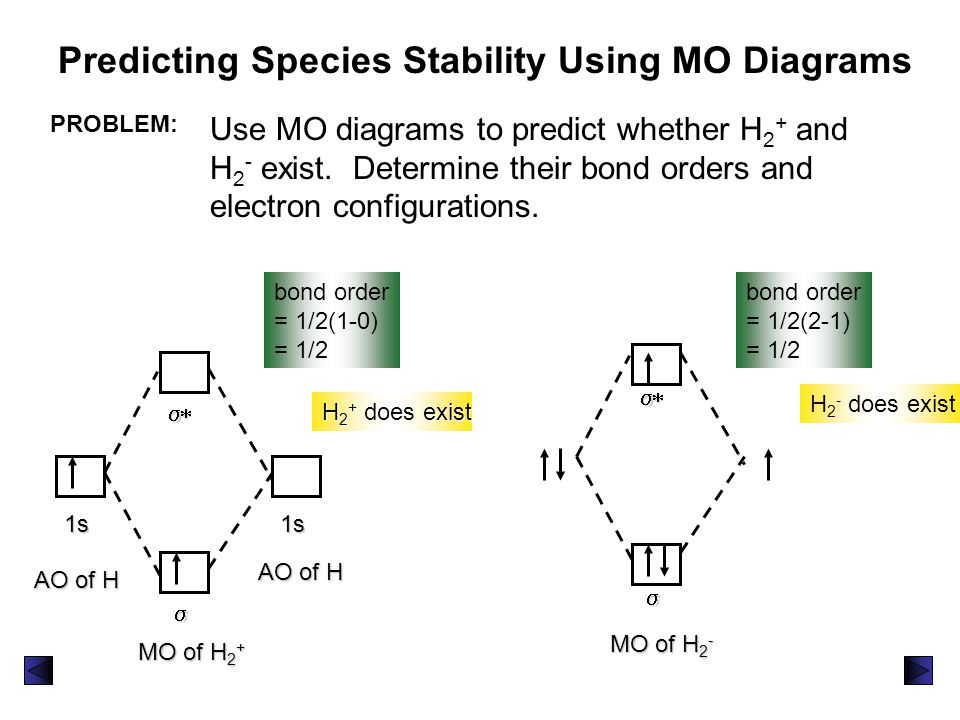
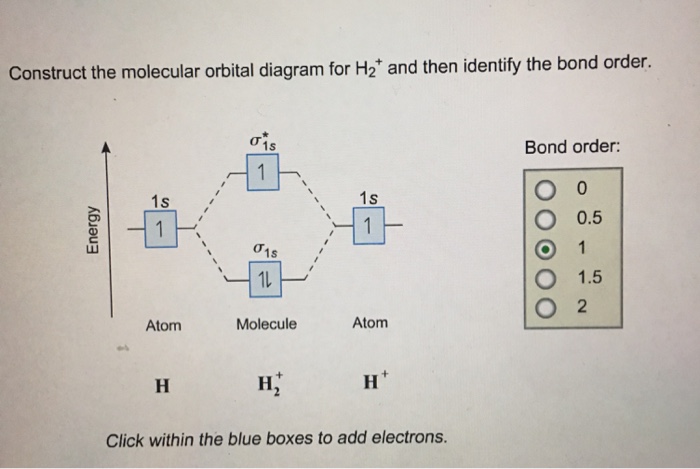
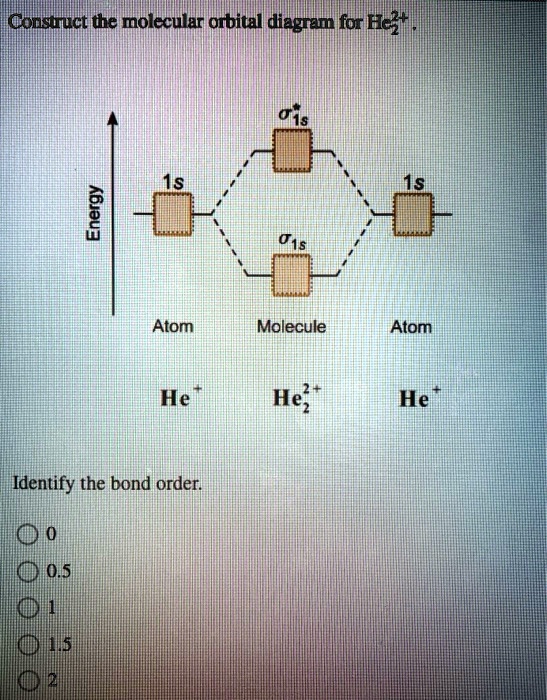
Draw the molecular orbital diagram of N2 and calculate the ... Draw the molecular orbital diagram of N2 and calculate the bond order. Answer: The bond order shows the number of chemical bonds present between a pair of atoms. The Bond Order Formula can be defined as half of the difference between the number of electrons in bonding orbitals and antibonding orbitals. Bond order formula is given as below. Complete An Mo Energy Diagram For H2+. - Wiring Diagrams A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.The Hydrogen Molecule Ion H2+Molecular Orbital Diagrams of Diatomic Molecules - Chem Solved A.Construct the molecular orbital diagram for H2^2 ... This problem has been solved! A.Construct the molecular orbital diagram for H2^2+ and then identify the bond order. B.Construct the molecular orbital diagram for H2 and then identify the bond order. C.Construct the molecular orbital diagram for H2^- and then identify the bond order. D.Construct the molecular orbital diagram for H2^+ and then ... How do I calculate the bond order for H2- and H2+? | Socratic Each hydrogen atom contributes one electron, and thus, H− 2 has three electrons while H+ 2 has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one σ1s and one σ* 1s MO by conservation of orbitals. If you calculate their bond order, you get: BOH+ 2 = 1 2(Bonding − Antibonding)
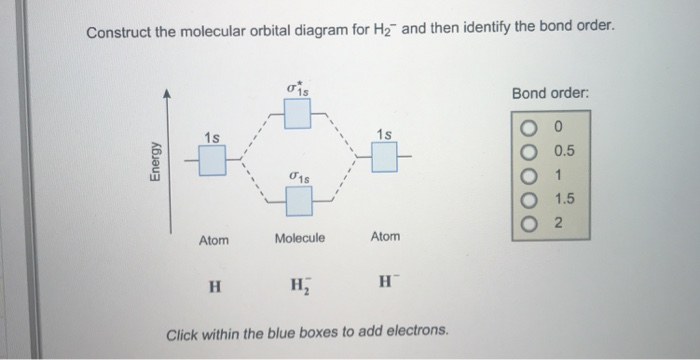
Construct the molecular orbital diagram fo... | Clutch Prep Problem: Construct the molecular orbital diagram for H2- and then identify the bond order. Click thin the blue boxes to add electrons.Bond order: a) 0 b) 0.5c) 1 d) 1.5e) 2
Molecular Orbital Diagram For He2 The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic orbital give total of 4 electrons in this molecule. Two are placed in the bonding orbital, the other two in antibonding orbital. The bond order = 1/2 x (Number of Bonding Electrons - Number of Antibonding Electrons) = .
Construct The Molecular Orbital Diagram For He2 And Then ... 1. Problem: Draw MO energy diagrams for the molecular ions H2+ and H Since both molecular ions have a bond order of 1/2, they are approximately equally.Solution: Construct the molecular orbital diagram for He2 + and then identify the bond order. Problem Construct the molecular orbital diagram for He 2 + and then identify the bond order.
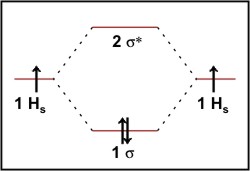
What is Bond order of H2? | UO Chemists The MOT diagram for H2 The overlap of 1s atomic orbitals forms H 2. Its shell has two molecular orbitals. one is bonding (σ 1s ), and the other is antibonding (σ *1s ). The H 2 molecule has two electrons, which can be accommodated in the σ 1s molecular orbital. Following the Pauli exclusion principle, this electron must have opposite spins.
Molecular orbital diagram of h2 - fornoob.com Molecular orbital diagram of hydrogen molecule: Bond order: From the molecular orbital diagram, there are 2 electrons in bonding molecular orbital and there is no anti - bonding molecular orbital. The bond order can be determined by substituting those values using bond order formula. Take care while calculating the bond order.
According to molecular orbital theory, what is the bond ... To determine the bond order of oxygen, we need to first draw its molecular orbital energy diagram, oxygen contains 12 valence electrons. Its orbital diagram is sigma to S. Than sigma to a star sigma to pEE, P two, P P two P star and then sigma two P star. We plug in the 12 valence electrons according to the off Bob Principle, the lowest energy molecular orbital first.
Answered: construct the molecular orbital diagram… | bartleby construct the molecular orbital diagram for H2− and then identify the bond order. Question. construct the molecular orbital diagram for H2− and then identify the bond order. Expert Solution. Want to see the full answer? Check out a sample Q&A here. See Solution. Want to see the full answer?
He2 2+ Molecular Orbital Diagram - schematron.org the molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the h 2 molecule is shown in figure on either side of the central ladder are shown the energies of the 1 s orbitals of atoms a and b, and the central two-rung ladder shows the energies of the bonding and antibonding.the …
Consider The Molecular Orbital Diagram For The H2, Shown ... Construct the molecular orbital diagram for H2 and then identify the bond order. 1S Bond order: 1s 1s O 0.5 1s O 1.5 2 Atom Molecule Atom Click within the blue boxes to add electrons.
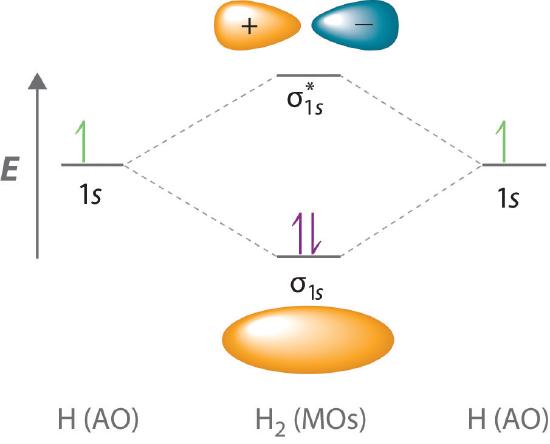
7.7 Molecular Orbital Theory - Chemistry Fundamentals A molecular orbital can hold two electrons, so both electrons in the H 2 molecule are in the [latex]\sigma[/latex] 1s bonding orbital; the electron configuration is [latex]{\left({\sigma}_{1s}\right)}^{2}.[/latex] We represent this configuration by a molecular orbital energy diagram (Figure 7.7.10) in which a single upward arrow indicates one ...
PDF Simple Molecular Orbital Theory - University of California ... LCAO MO Energy Diagram for H2 Energy H-H ∆E1 ∆E2 • ∆E2> ∆E1, so the antibonding orbital is always more anti-bonding than the bonding orbital is bonding H2molecule: two 1s atomic orbitals combine to make one bonding and one antibonding molecular orbital. Ha Hb
Is H2 a viable molecule for the molecular orbital ... - Quora Answer (1 of 2): According to the molecular orbital theory, a molecular is viable of its bond order is more than or equal to one. The bond order is defined as number of bond between to two atoms of that molecule. It is calculated as the difference of electrons in bonding molecules and anti-bondin...
Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in .
Solved Construct the molecular orbital diagram for H2- and ... Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Question: Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons.

















0 Response to "39 molecular orbital diagram for h2- and bond order"
Post a Comment