41 phase change diagram with equations
Phase Change Diagram Worksheet Answers - kidsworksheetfun A phase diagram is a graphical way to depict the effects of pressure and temperature on the phase of a substance. 1 80 phæe change 60 1 40 1 20 100 60 40 12 tim e minutes 20 22 at point a the beginning of observations the substance exists in a solid state. Phase diagram worksheet name. PDF Phase Diagrams and Phase Separation - University of Cincinnati Phase Diagrams This common tangent construction can be extended to quite complicated situations, with several minima, which then give rise to quite complicated free energy curves and hence phase diagrams. For plotting a phase diagram we need to know how solubility limits (as determined by the common tangent construction) vary with temperature.
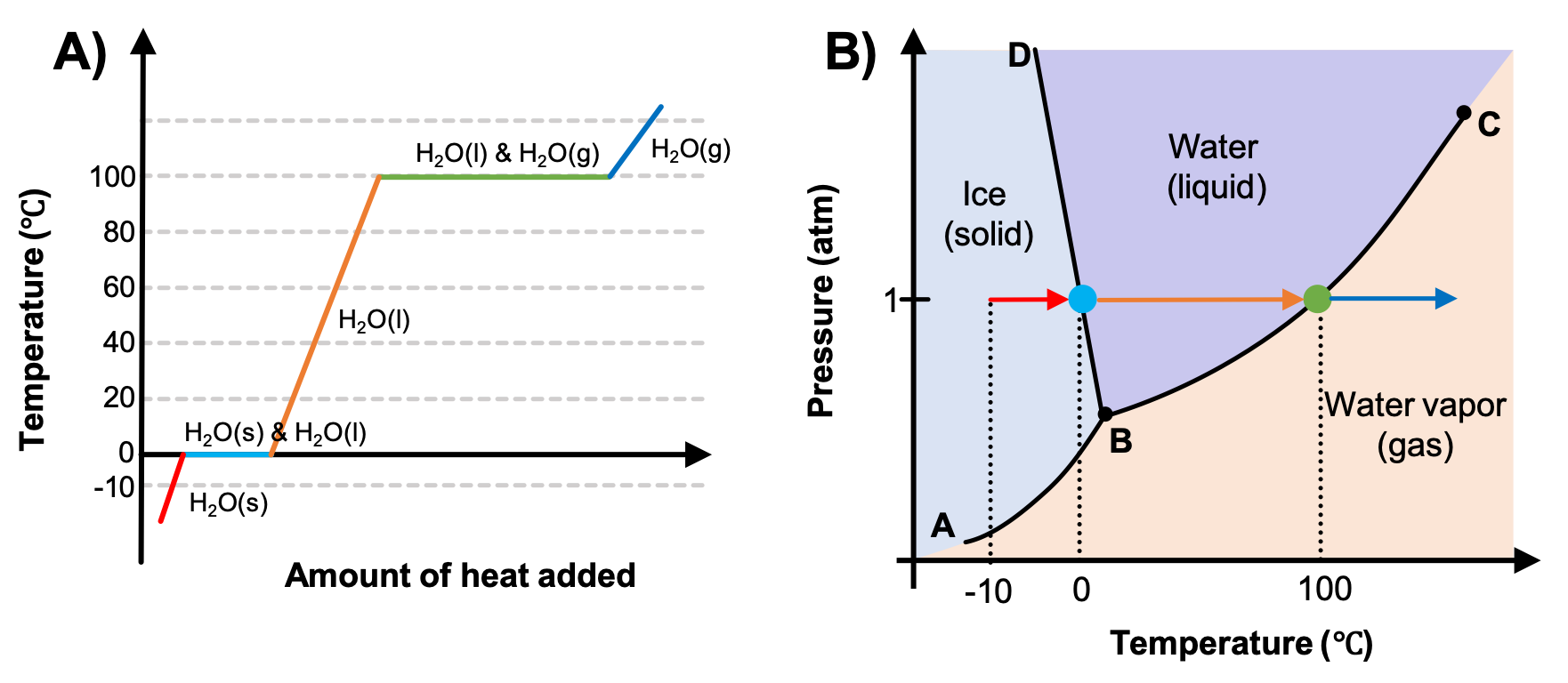
Heating Curves and Phase Diagrams (M11Q2) – UW-Madison ... Phase diagrams (plots of pressure vs. temperature) were correlated with heating curves (plots of temperature vs. energy). These two types of plots provide complementary information on the phase transitions of substances. While a heating curve provides information on the phase changes at a single pressure, the phase diagram depicts the phase ...

Phase change diagram with equations
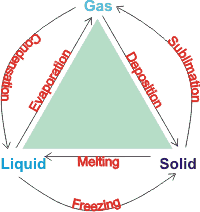
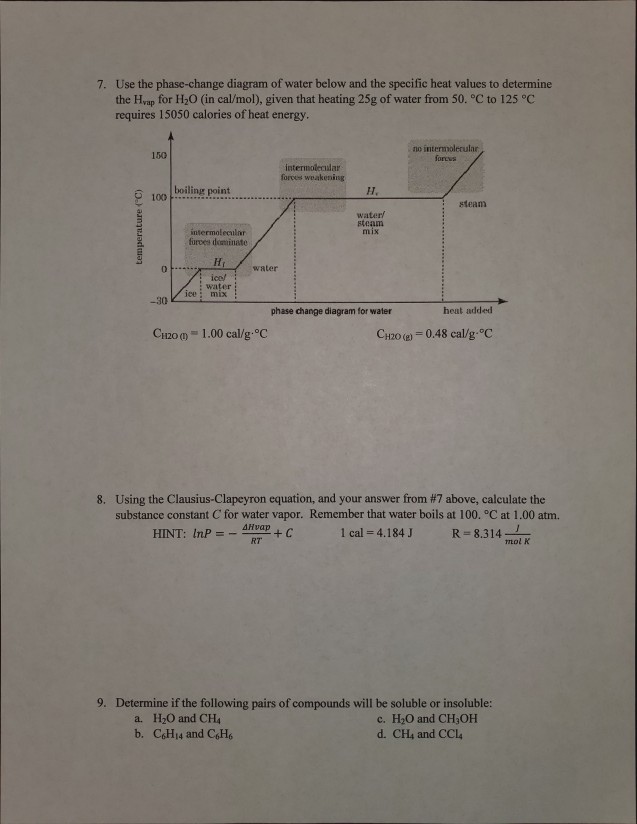
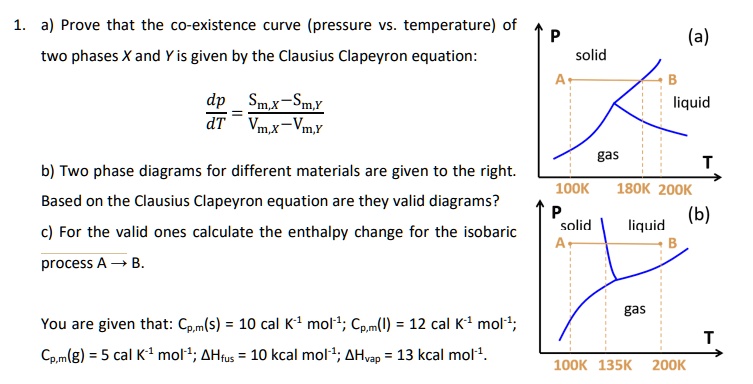
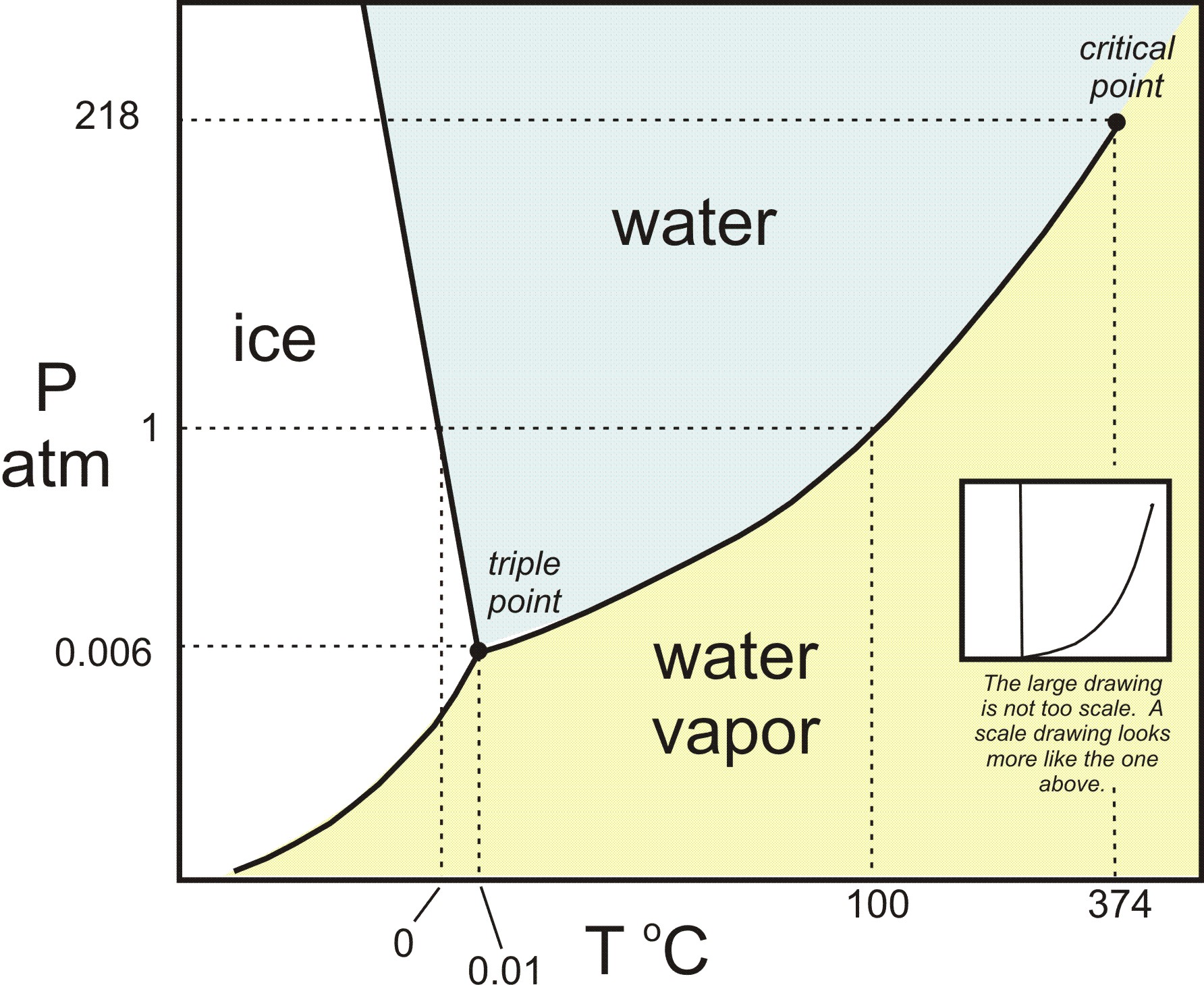
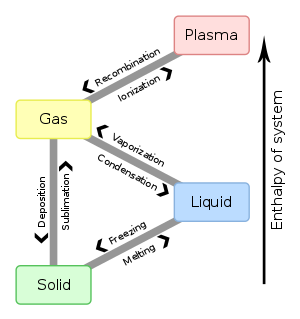
3.3 Phase Diagram for Water Vapor: Clausius Clapeyron ... 3.3 Phase Diagram for Water Vapor: Clausius-Clapeyron Equation. The Clausius-Clapeyron Equation. We can derive the equation for e s using two concepts you may have heard of and will learn about later: entropy and Gibbs free energy, which we will not go into here.Instead, we will quote the result, which is called the Clausius-Clapeyron Equation, 10.3 Phase Change Diagrams | Chemistry - Lumen Learning Heat water from 0 °C to 100 °C. Boil water. Heat steam from 100 °C to 120 °C. The heat needed to change the temperature of a given substance (with no change in phase) is: q = m × × c × × Δ T (see previous chapter on thermochemistry). The heat needed to induce a given change in phase is given by q = n × × Δ H. Phase Change or Phase Transition - Definition & Examples Condensation. Condensation is the process by which, the physical state of a substance changes from its gas phase to the liquid phase. It can also be defined as the transition of water vapour into water droplets, upon contacting a solid surface. This process is useful in separating a solute and solvent from its solution.
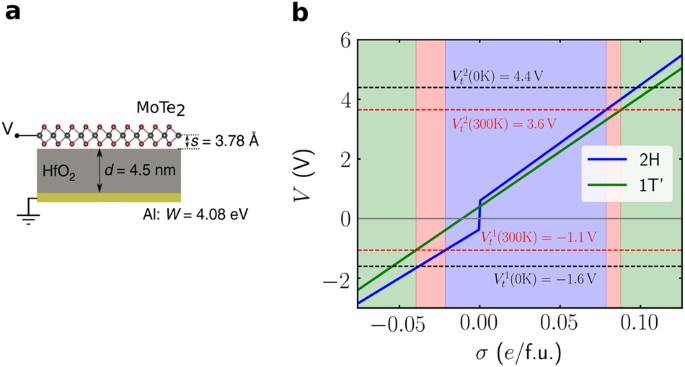
Phase change diagram with equations. Equations of State and Phase Diagrams - UNAM Equilibria between two phases are represented by a (two- dimensional) curve within the three-dimensional diagram, and equilibrium among three phases is represented by a straight line situated along the constant temperature–pressure equilibrium condition. Figure 2 shows that the triple “point” of a pT PDF Thermodynamics and Phase Diagrams A phase diagram is a graphical representation of the values of the thermodynamic variables when equilibrium is established among the phases of a system. Materials scientists are most familiar with phase diagrams which involve temperature, , and T composition as variables. Examples are T-composition phase diagrams for binary How can I calculate thermochemistry equations for phase ... The equation used is q = m⋅ ΔH, where ΔH - the heat required for one gram of the substance to undergo the phase change. If you're going from solid to liquid, you use ΔH fusion (called the heat of fusion ), which represents the heat required for 1 gram of substance to change from solid to liquid at melting point. Clapeyron Equation - Teaching Phase Equilibria The Clapeyron equation (also called the Clausius-Clapeyron equation) relates the slope of a reaction line on a phase diagram to fundamental thermodynamic properties. What is the Clapeyron Equation? The reaction brucite = periclase + H2O. This reaction has a typical shape for a dehydration (or decarbonation reaction). Figure from D. Perkins.
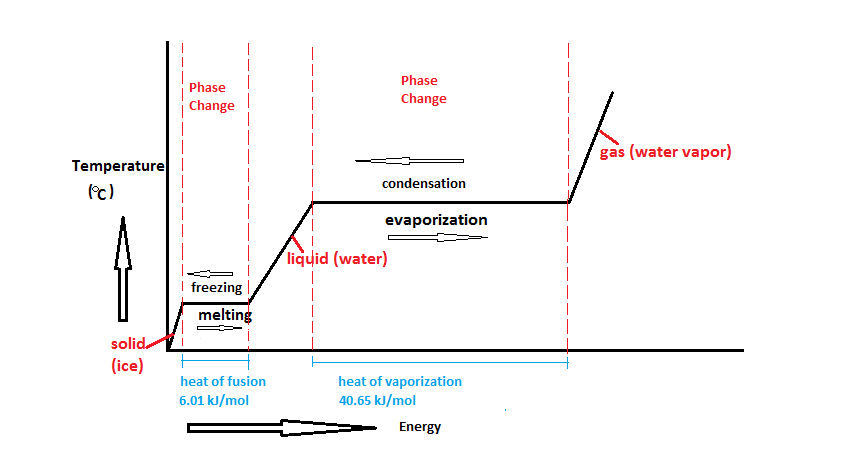
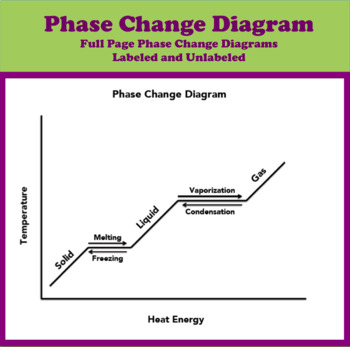
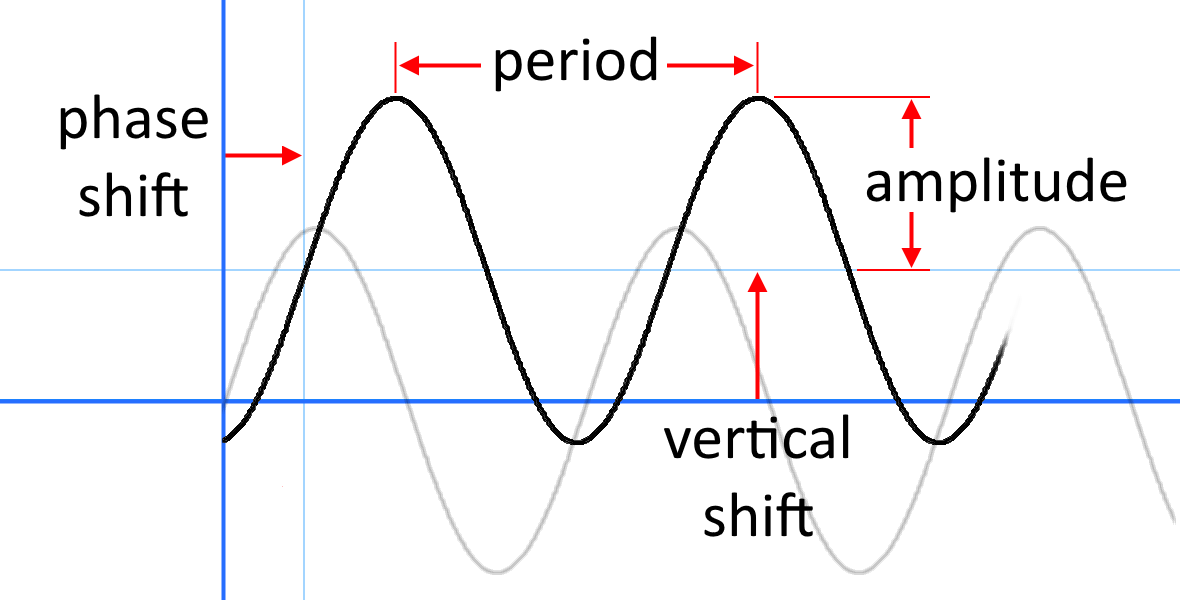
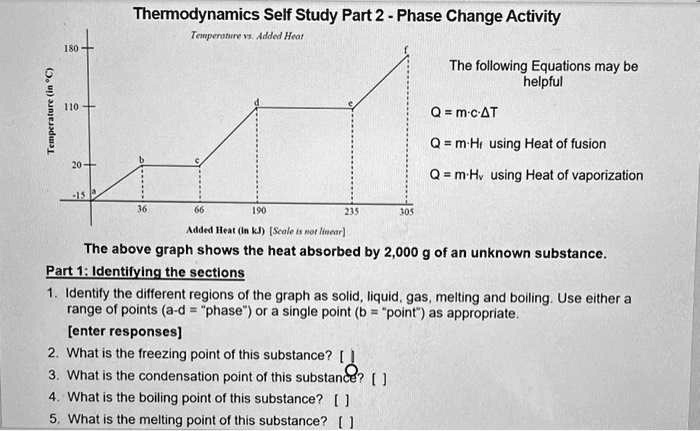
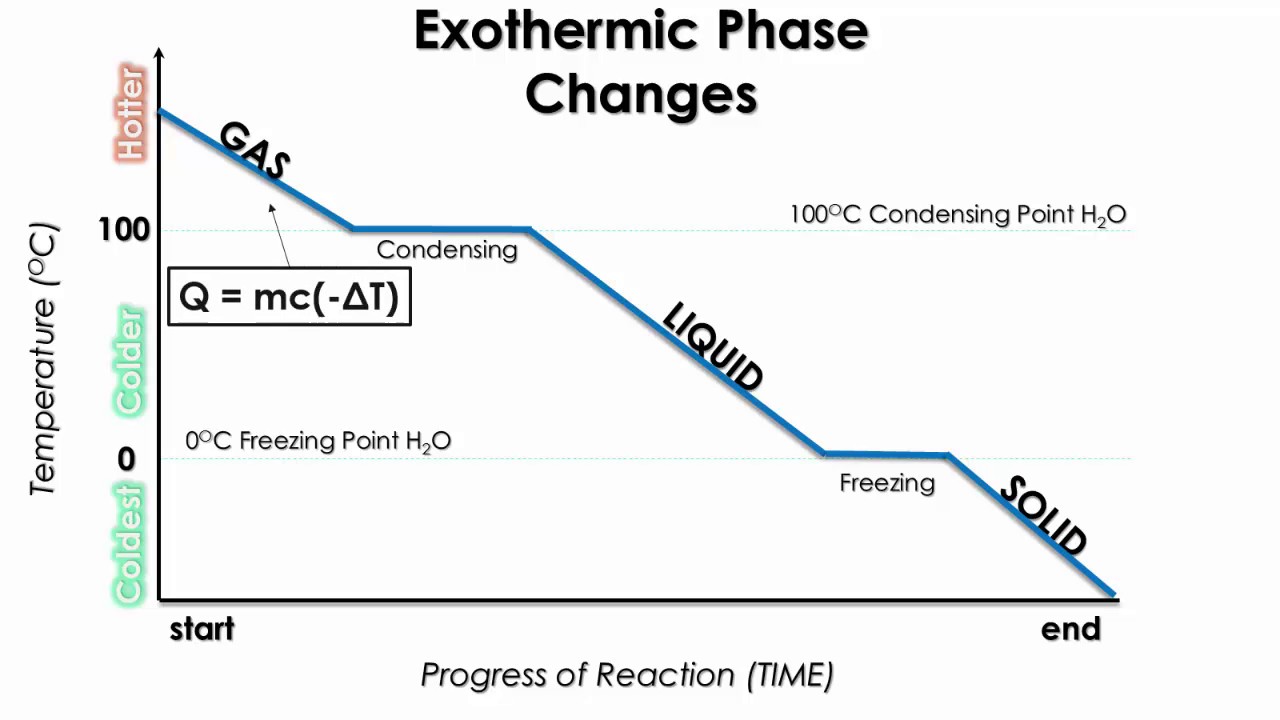
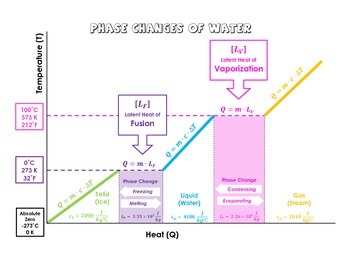
DOC Phase Changes Worksheet - Boyertown Area School District Label this on the diagram. (40.7 kJ per mol for water) Phase Change Diagram. The graph was drawn from data collected as 1 mole of a substance was heated at a constant rate. Use the graph to answer the following questions. Use the phase change diagram above to answer the following questions. Describe what is occurring from; A to B. B to C. C to ... PDF 1.5Phase LineandBifurcationDiagrams - University of Utah The phase line diagram has a close relative called a bifurcation dia-gram. The purpose of the diagram is to display qualitative information about equilibria, across all equations y′ = f(y), obtained by varying physical parameters appearing implicitly in f. In the simplest cases, each parameter change to f(y) produces one phase line diagram and Phase Diagram Worksheet - Studying Worksheets Phase change diagram practice worksheet answers. 407 kJ per mol for water Phase Change Diagram The graph was drawn from data collected as 1 mole of a substance was heated at a constant rate. Which section represents the solid phase. PDF 1.5 Phase Line and Bifurcation Diagrams A phase line diagram for the autonomous equation y0= f(y) is a line segment with labels sink, source or node, one mark and label for each root yof f(y) = 0, i.e., each equilibrium; see Figure 15. A phase line diagram summarizes the contents of a direction eld and all equilibrium solutions. It is used to draw threaded curves across the graph window,
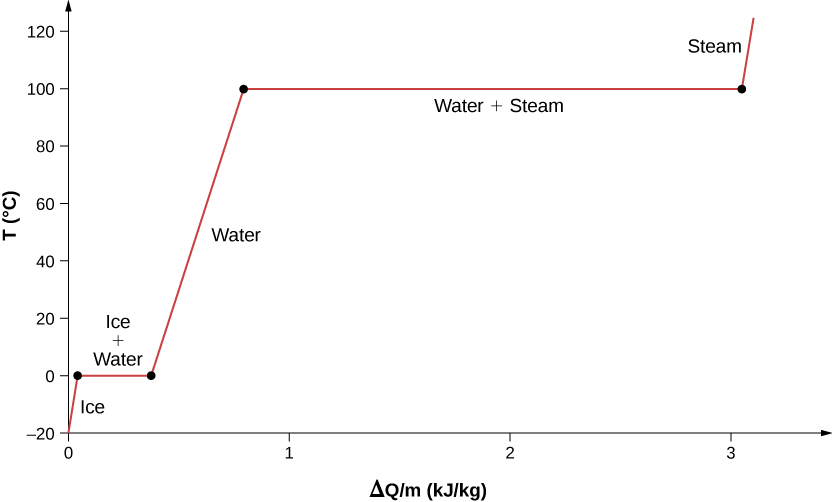
11.3 Phase Change and Latent Heat - Physics | OpenStax A phase diagram indicating the temperature changes of water as energy is added is shown in Figure 11.10. The ice starts out at −20 °C, and its temperature rises linearly, absorbing heat at a constant rate until it reaches 0 °. Once at this temperature, the ice gradually melts, absorbing 334 kJ/kg. The temperature remains constant at 0 PDF Heat Transfer with Phase Change Heat Transfer with Phase Change So far we have discussed heat transfer at a boundary due to a temperature difference between bulk temperatures x bw q hT T A 1. forced convection • laminar • turbulent 2. natural convection 3. phase change When a phase change takes place, the temperature on one Phase Diagrams – Chemistry Use phase diagrams to identify stable phases at given temperatures and pressures, and to describe phase transitions resulting from changes in these properties. In the previous module, the variation of a liquid’s equilibrium vapor pressure with temperature was described. Considering the definition of boiling point, plots of vapor pressure ... Differential Equations - Phase Plane - Lamar University In this section we will give a brief introduction to the phase plane and phase portraits. We define the equilibrium solution/point for a homogeneous system of differential equations and how phase portraits can be used to determine the stability of the equilibrium solution. We also show the formal method of how phase portraits are constructed.
Phase Change: Evaporation, Condensation, Freezing, Melting ... Aug 27, 2021 · Phase change is often shown in a diagram like the one below: Diagram of water phase changes When a substance is in a solid state, it can absorb a lot of energy in the form of heat until it hits ...
3.4 Solving Energy Problems Involving Phase Changes and ... 3.4 Solving Energy Problems Involving Phase Changes and Temperature Changes. When a cloud drop evaporates, the energy to evaporate it must come from somewhere because energy is conserved according to the 1 st Law of Thermodynamics. It can come from some external source, such as the sun, from chemical reactions, or from the air, which loses some energy and thus cools.
PDF Generating Complex Phase Diagrams Using Regular Solution ... Using regular solution theory, create a temperature vs. composition phase diagram of solid-liquid equilibria of a binary mixture. Use this phase diagram, to design a continuous process that precipitates out solid of a specified composition. Computational Objectives: Apply the ability to numerically solve systems of nonlinear algebraic equations and
1.5 Phase Changes - General Physics Using Calculus I The heat Q absorbed or released in a phase change in a sample of mass m is given by Q = mLf(melting/freezing) (1.7) (1.7) Q = m L f (melting/freezing) Q = mLv(vaporization/condensation) (1.8) (1.8) Q = m L v (vaporization/condensation)
PDF Phase Plane Diagram PHASE PLANE DIAGRAM Graphics Commands 2-164 March 10, 1997 DATAPLOT Reference Manual PHASE PLANE DIAGRAM PURPOSE Generates a phase plane diagram. DESCRIPTION A first order differential equation is one of the form: y'=F(y,t) where t is an independent variable (usually time), y is a dependent variable, and y' is the derivative of y.
Phase Change Diagram Worksheet Pdf - Worksheet Smart Phase change diagram worksheet doc. The graph was drawn from data collected as a substance was heated at a constant rate. Phase Diagram Worksheet Answers One Step Equations Algebra Equations Worksheets Equations . Phases Of Matter Phase Introduction And Phase Change Activity Matter Activities Matter Activities
how does a phase change affect a thermochemical equation ... A thermochemical equation has two parts: a balanced chemical equation and the change in one or more thermodynamic quantities (e.g., temperature, energy, or enthalpy) that occurs when that change occurs. The balanced equation can describe either a physical change (as in the example shown) or a chemical change.
PDF Phase Plane Diagrams of Difference Equations System of Difference Equations x(k +1) = ax(k)+by(k) y(k +1) = cx(k)+dy(k) General solution: z(k) = Akz(0) Where z = x(k) y(k) and A = a b c d Dewland, Weston, Weyrens Phase Plane Diagrams of Difference Equations Introduction Phase Plane Diagrams Example Conclusion Terminology Affine Transformation
Phase Changes - University Physics Volume 2 Phase Diagrams. The phase of a given substance depends on the pressure and temperature. Thus, plots of pressure versus temperature showing the phase in each region provide considerable insight into thermal properties of substances. Such a pT graph is called a phase diagram. shows the phase diagram for water. Using the graph, if you know the ...
PHASE PLANE DIAGRAMS OF DIFFERENCE EQUATIONS We say the phase portraits of z(k+1) = Az(k) and z(k+1) = Jz(k) are a ne equivalent if Aand Jare similar. To study the phase portrait of z(k+1) = Az we can consider the a ne transformation w(k+ 1) = Jw, where J is a 2 2 matrix that has a particularly simple form. Let P be a 2 2 invertible matrix.
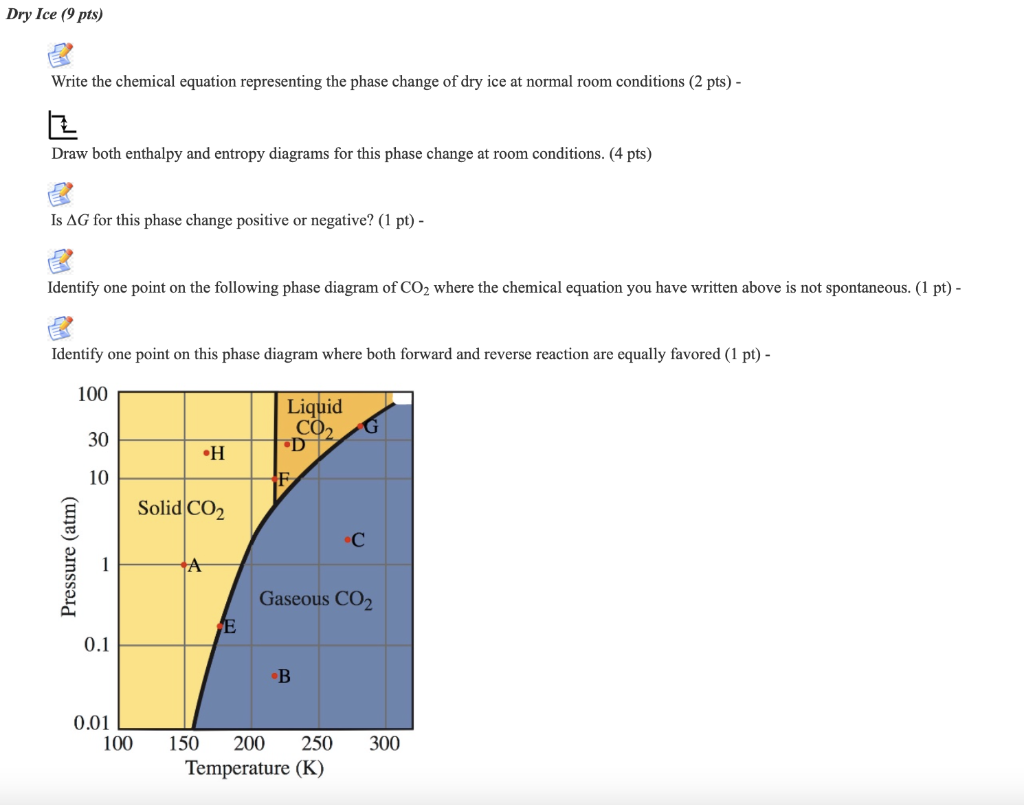
PDF Phase Changes and the Equation of State of Zr Phase Changes and the Equation of State of Zr 2 1. Introduction The phase diagrams of elements are fundamental data for the physics of materials. Significant attention has focused on phase ...
Phase Change or Phase Transition - Definition & Examples Condensation. Condensation is the process by which, the physical state of a substance changes from its gas phase to the liquid phase. It can also be defined as the transition of water vapour into water droplets, upon contacting a solid surface. This process is useful in separating a solute and solvent from its solution.
10.3 Phase Change Diagrams | Chemistry - Lumen Learning Heat water from 0 °C to 100 °C. Boil water. Heat steam from 100 °C to 120 °C. The heat needed to change the temperature of a given substance (with no change in phase) is: q = m × × c × × Δ T (see previous chapter on thermochemistry). The heat needed to induce a given change in phase is given by q = n × × Δ H.
3.3 Phase Diagram for Water Vapor: Clausius Clapeyron ... 3.3 Phase Diagram for Water Vapor: Clausius-Clapeyron Equation. The Clausius-Clapeyron Equation. We can derive the equation for e s using two concepts you may have heard of and will learn about later: entropy and Gibbs free energy, which we will not go into here.Instead, we will quote the result, which is called the Clausius-Clapeyron Equation,























0 Response to "41 phase change diagram with equations"
Post a Comment