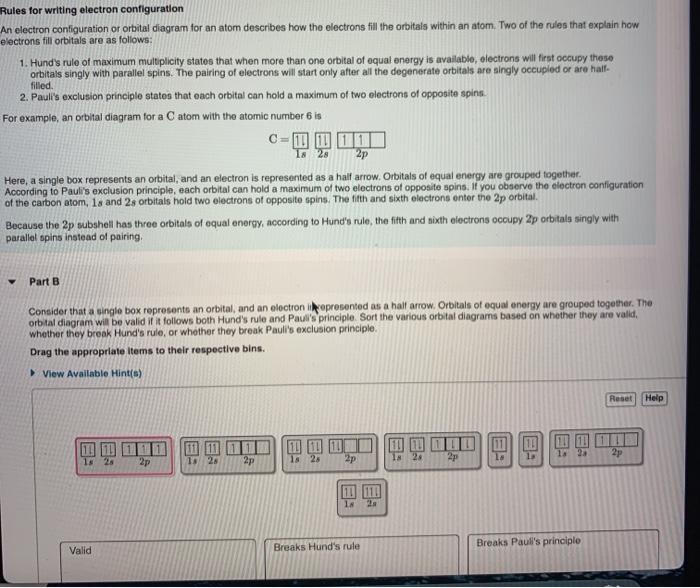
40 what is the maximum number of electrons that can occupy a box in an orbital filling diagram
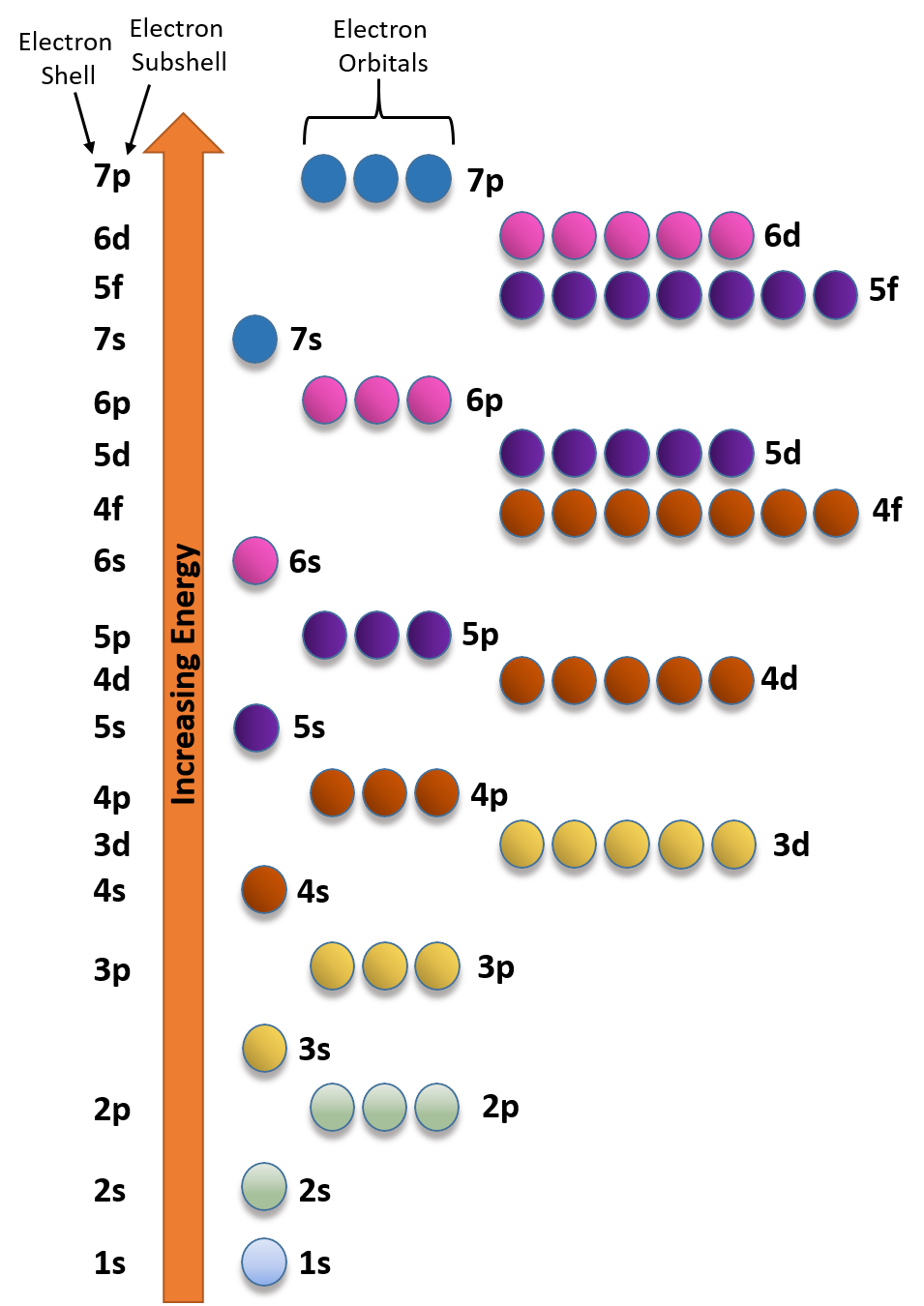
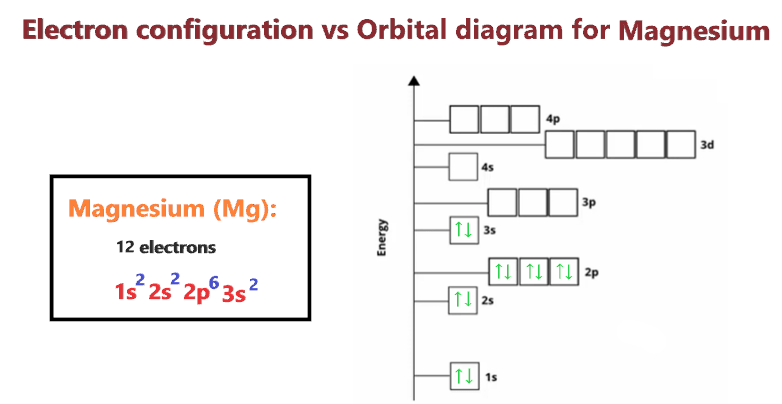
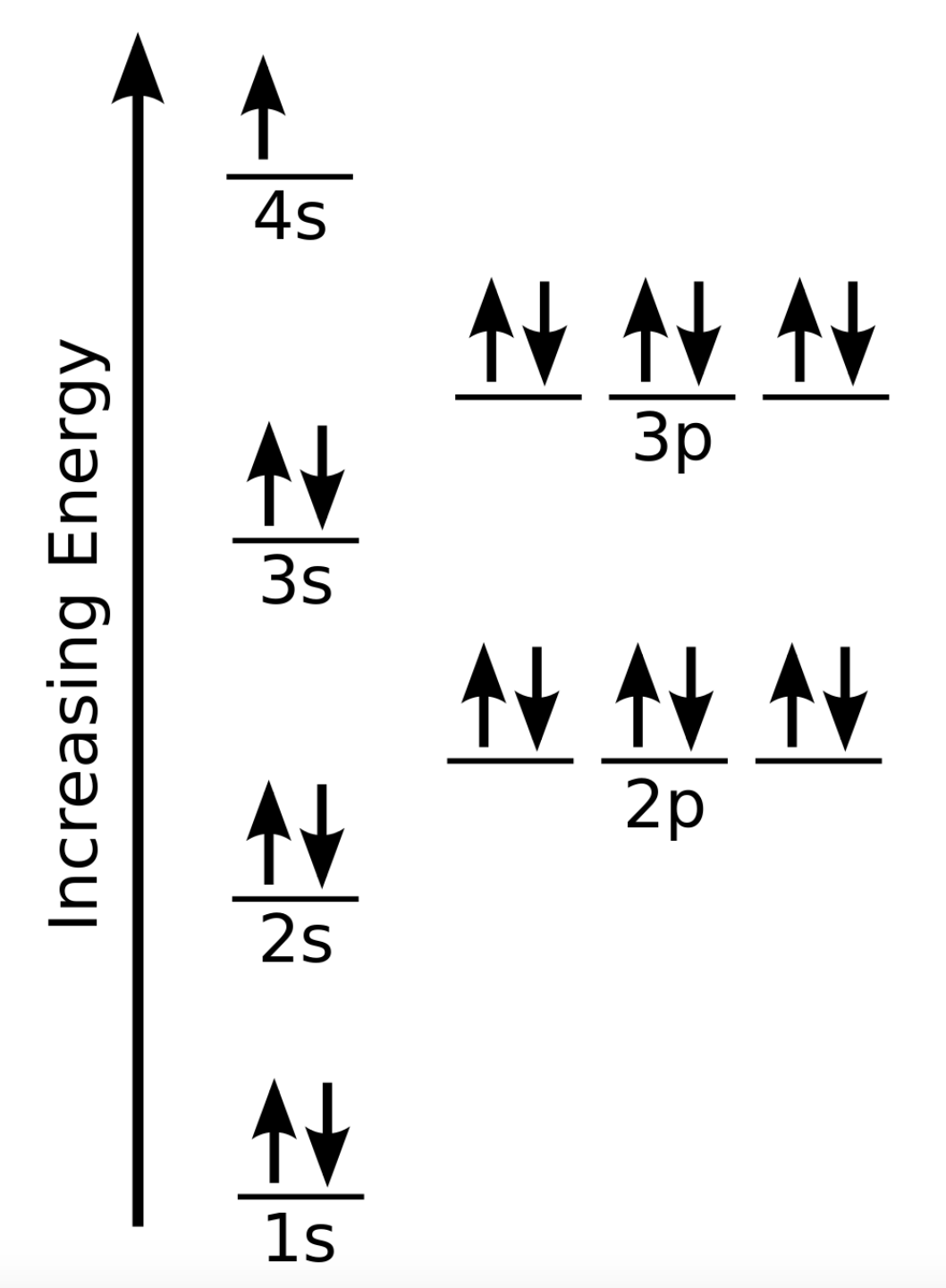
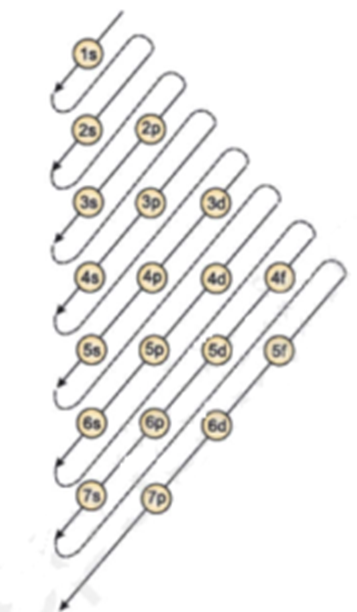
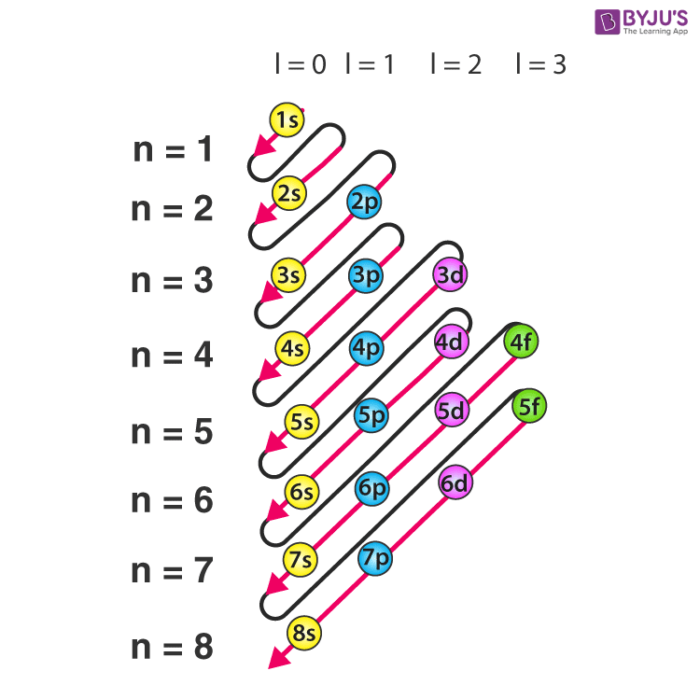
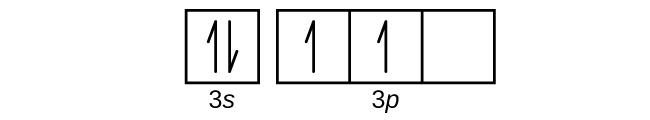
Which Quantum Number Describes the Shape of an Orbital For every value of n there is one s orbital ie. In chemistry and spectroscopy ℓ 0 is called an s orbital ℓ 1 a p orbital ℓ 2 a d orbital and ℓ 3 an f orbital. What is the maximum number of electrons that can occupy a box in an orbital filling diagram. An _____ defines a two-dimensional shape. Size of the orbital. Azimuthal quantum number ie. Electron Configurations and Orbital Box Diagrams ... An orbital box diagram can be written as well. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin-one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for the first 20 elements in the figure below.
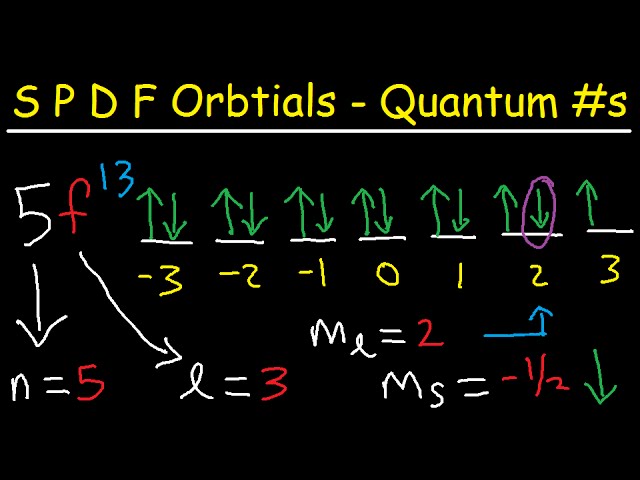
M7Q7: Electron Configurations, Orbital Box Notation - Chem ... An atom of boron (atomic number 5) contains five electrons. The n = 1 shell is filled with two electrons and three electrons will occupy the n = 2 shell. Because any s subshell can contain only two electrons, the fifth electron must occupy the next energy level, which will be a 2p orbital. There are three degenerate 2p orbitals (m l = −1, 0, +1) and the electron can occupy any one of these p ...

What is the maximum number of electrons that can occupy a box in an orbital filling diagram
Atom Questions and Answers - Study.com What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? View Answer Using an orbital diagram, determine the number of unpaired electrons in ... © What is the maximum number of electrons that can occupy ... Register Now. Lorem ipsum dolor sit amet, consectetur adipiscing elit.Morbi adipiscing gravdio, sit amet suscipit risus ultrices eu.Fusce viverra neque at purus laoreet consequa.Vivamus vulputate posuere nisl quis consequat. Boron Orbital diagram, Electron configuration, and Valence ... P orbital contains 3 boxes that can hold a maximum of 6 electrons. D orbital contains 5 boxes that can hold a maximum of 10 electrons. F orbital contains 7 boxes that can hold a maximum of 14 electrons. The orbital diagram will also be filled with the same order as described by the Aufbau principle. (1s < 2s < 2p < 3s……and so on.)
What is the maximum number of electrons that can occupy a box in an orbital filling diagram. PDF What is the maximum number of electrons in the 4d subshell The n = 1 shell is filled with two electrons and three electrons will occupy the n = 2 shell. Because any s subshell can contain only two electrons, the fifth electron must occupy the next energy level, which will be a 2p orbital. There are three degenerate 2p orbitals (ml = −1, 0, +1) and the electron can occupy any one of these p orbitals. Rank the following particles, from largest to smallest in ... It has 7 electrons in it last shell. it will gain one electron to complete its octet and become Cl-. The ionic bond of chlorine form with potassium is Kcl ( potassium chloride) Phosphorus has 5 electrons in its last shell it need 3 more electron to stable . Sulphur has 6 electron. it needs 2 electrons Chemistry 2.12 Quiz - Chemistry 2.12 Quiz: Electron ... Chemistry 2.12 Quiz: Electron Orbitals ion 1 1 / 1 point What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? 8 2 6 14 View Feedback ion 2 1 / 1 point Look at an aufbau diagram. Chemistry 2.12: Electron Orbitals Flashcards | Quizlet Chemistry 2.12: Electron Orbitals. What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? What is the maximum number electrons that can occupy any d orbital? Use an aufbau diagram.
Orbital Diagram Practice | Chemistry Quiz - Quizizz Orbital diagram. Tags: Question 12. SURVEY. 300 seconds. Q. The electron configuration of an atom is 1s 2 2s 2 2p 6. The number of electrons in the atom is. answer choices. What Is The Maximum Number Of Electrons That Can Occupy A ... Each shell can contain only a fixed number of electrons: The first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. The general formula is that the nth shell can in principle hold up to 2 (n 2) electrons. (Visited 26 times, 1 visits today) Question What must be done to the refrigerant that is vented off ... When filling degenerate orbitals, electrons fill them singly first, with parallel spins is known as; What is the maximum number of electrons that can occupy a box in an orbital filling diagram; Elements that are characterized by the filling of p orbitals; A(n) ____ contains instructions for filling areas of a template. How to write Electron Configuration for any element | All ... S orbital contains 1 box that can hold a maximum of 2 electrons. P orbital contains 3 boxes that can hold a maximum of 6 electrons. D orbital contains 5 boxes that can hold a maximum of 10 electrons. F orbital contains 7 boxes that can hold a maximum of 14 electrons. The orbital diagram will also be filled with the same order as described by ...
What is the maximum number of electrons that can occupy a ... 🔴 Answer: 3 🔴 on a question What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? - the answers to ihomeworkhelpers.com PDF How many electrons can occupy any single subshell orbital What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? 2. Consult an aufbau diagram. What is the maximum number of electrons that can occupy an orbital quizlet? Each orbital can hold a maximum of 2 electrons, and only if the electrons have opposite spins (Pauli exclusion principal). Hund's Rule and Orbital Filling Diagrams | Chemistry for ... There are two 2 p electrons for carbon and each occupies its own 2 p orbital. Figure 3. Orbital filling diagram for carbon. Oxygen has four 2 p electrons. After each 2 p orbital has one electron in it, the fourth electron can be placed in the first 2 p orbital with a spin opposite that of the other electron in that orbital. Figure 4. 2.12 Lesson Assessment: Electron Orbitals Flashcards - Quizlet What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? → 6 → 14 → 8 → 2 2 Look at an aufbau diagram. What is the maximum number electrons that can occupy any d orbital? → 14 → 6 → 4 → 10 10 What electrically neutral atom will have an electron figuration of 1s²2s²2p? Use a periodic table.
The __________ quantum number defines the shape of an orbital. In a px orbital, the subscript x denotes the _____ of the electron. To insert a shape, click a shape in the shapes ____. What is the maximum number of electrons that can occupy a box in an orbital filling diagram; The principal quantum number indicates what property of an electron? The angular momentum quantum number is 3 in _____ orbitals.

what is the maximum number of electrons that can occupy a d energy sublevel none of the above whal is the maximum number of electrons hat can occupy he znd energy level none of the above 10 67357
What is the maximum number of electrons that can occupy a ... oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in …
What is the maximum number of electrons in the 2p sub ... The 2p sub-level can hold a maximum of 6 electrons. sub-level is divided into 2px, 2py and 2pz. Each of those orbitals can hold a maximum of 2 electrons. There are 3 of them and thus 6 total for...
s,p,d,f Orbitals - Chemistry | Socratic Not all electrons inhabit s orbitals. At the first energy level, the only orbital available to electrons is the 1s orbital. However, at the second level, there are also orbitals called 2p orbitals in addition to the 2s orbital. Unlike an s orbital, a p orbital points in a particular direction. The one shown below points up and down the page.
Hunds Rule - Aufbau Principle | Pauli's Exclusion Principle The p orbital can hold a maximum number of 6 electrons, 'd' orbital can hold a maximum number of 10 electrons and the f orbitals can hold a maximum number of 14 electrons in the orbital shell. For Example: Chlorine 17. 1s 2 2s 2 2p 6 3s 2 3p 5. In the above distribution of electrons, orbitals in which s orbital can hold a maximum of 2 electrons ...
What is the maximum number of electrons that can occupy a ... Correct answers: 3 question: What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level?
What is the orbital notation for chromium? - Answers Shorthand orbital notation is written as the orbital with the number of electrons in that orbital in superscript. For example, if 2p has 4 electrons in it is written as 2p^4. How is an unoccupied ...
Boron Orbital diagram, Electron configuration, and Valence ... P orbital contains 3 boxes that can hold a maximum of 6 electrons. D orbital contains 5 boxes that can hold a maximum of 10 electrons. F orbital contains 7 boxes that can hold a maximum of 14 electrons. The orbital diagram will also be filled with the same order as described by the Aufbau principle. (1s < 2s < 2p < 3s……and so on.)
© What is the maximum number of electrons that can occupy ... Register Now. Lorem ipsum dolor sit amet, consectetur adipiscing elit.Morbi adipiscing gravdio, sit amet suscipit risus ultrices eu.Fusce viverra neque at purus laoreet consequa.Vivamus vulputate posuere nisl quis consequat.
Atom Questions and Answers - Study.com What is the maximum number of electrons that can occupy a box in an orbital filling diagram at any energy level? View Answer Using an orbital diagram, determine the number of unpaired electrons in ...




















0 Response to "40 what is the maximum number of electrons that can occupy a box in an orbital filling diagram"
Post a Comment