40 fluorine electron dot diagram
Lewis Dot Diagram For Fluorine Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?). A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Lewis Dot Diagram For Fluorine - schematron.org In Lewis diagrams the atoms are shown by writing the atomic symbol surrounded by one dot for each of the valence electrons. In a covalently bound molecule the dots are arranged in pairs, with the bound pairs placed between the . Fluorine is in Group 17 of the Periodic Table.. And thus the neutral atom has 7 valence electrons.
Electron Dot Structure For Fluorine - fluorine f atom ... Electron Dot Structure For Fluorine - 18 images - 30 bohr diagram of fluorine wiring diagram database, basic chemistry tutorial 4 ionic bonds sciencemusicvideos, life in nus, cbse class 10 chemistry draw the electron dot structures for,

Fluorine electron dot diagram
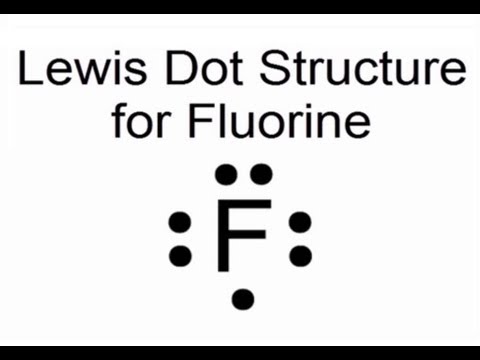
Lewis Dot Structure for Fluorine Atom (F) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for F (Fluorine). I show you where Fluorine is on the periodic table and how to determine ... Build an Atom - Atoms | Atomic Structure | Isotope ... - PhET Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas! SF6 Lewis Structure, Molecular Geometry ... - Techiescientist 2 days ago · In the outermost shell, we have 6 electrons that are also known as valence electrons in the case of Fluorine. Lewis Dot Structure of SF6. The central atom here is Sulfur as it is less electronegative than Fluorine. This is because the outer shell of Fluorine has 5 electrons and it needs one more electron to reach stability, which is easier to ...
Fluorine electron dot diagram. Nitrogen trifluoride (NF3) lewis dot structure ... - Topblogtenz Simple steps for drawing the NF3 lewis dot structure. 1. Count total valence electron in NF3. In the first step, we have to calculate the total number of valence electrons present in the NF3 molecule. Nitrogen is present in the 15th group in the periodic table and Fluorine in group 17th. ⇒ Total valence electron in Nitrogen = 5 Fluorine(F) electron configuration and orbital diagram Fluorine orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. 4. Draw a Lewis dot diagram of Lithium bonding with ... thamimspartan One lithium (Li) atom can combine with one fluorine (F) atom. Together, they make the formula LiF. Fluorine has seven electrons of it's own. Lithium gives up its one electron to make both atoms happy. So the fluorine atom has eight electrons, and a filled outer shell. An image is attached for referen Lewis Structure of TeF5- (With 5 Simple Steps to Draw!) Now, you can see in the above image that all the fluorine atoms form an octet. Also, only 40 valence electrons of TeF5- ion are used in the above structure.. But there are total 42 valence electrons in TeF5- ion (as calculated in step #1).. So the number of electrons left to be kept on the central atom = 42 - 40 = 2. So let's keep these two electrons (i.e 1 electron pair) on the central atom.
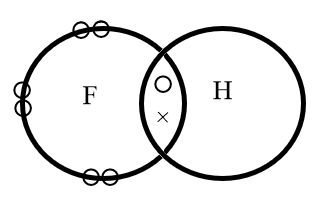
Fluorine Electron Dot Structure - the groups and electron ... Fluorine Electron Dot Structure - 15 images - exceptions to the octet rule boundless chemistry, fluorine atom structure, best overview is brf3 polar or nonpolar science, the smarticle particles taking representing compounds in, BeF2 lewis structure, Molecular geometry, Polar or ... Let's count the formal charge on the fluorine atom first, both fluorine atoms in the BeF2 Lewis structure ( 4th step) have the same bonded pair and lone pair, so, just count the F.C. for the one fluorine atom. For fluorine atom: ⇒ Valence electrons of fluorine = 7 ⇒ Lone pair electrons on fluorine = 6 Fluorine Bohr Model - How to draw Bohr diagram for ... Electron dot diagram of Fluorine atom The electron dot diagram also called lewis's structure which represents the valence electrons of atoms. As, from the Bohr diagram of Fluorine, we got to know, it has 7 valence electrons. So, just represent these 7 valence electrons around the Fluorine atom as a dot. The electron configuration of Fluorine Electron Dot Diagram For Fluorine - Wiring Diagrams Electron Dot Diagram For Fluorine A larger outer circle has one red dot on, representing the second shell with one electron. Lithium is in Group 1 of the Periodic Table. Fluorine. Structure of a. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?).
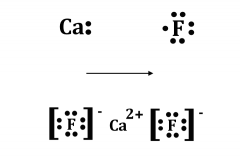
MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine Lewis Electron Dot Diagram For Fluoride Ion In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon.The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Examples. Lithium fluoride, LiF. Lithium atom loses one electron to form the cation Li+. Mark Scheme (Results - Edexcel Aug 22, 2018 · electron(s) and S gains electron(s) No M1 or M2 if mention of electron sharing or covalent bonding ALLOW Mg (ion) has a charge of 2+/+2 and S (ion) has a charge of 2-/-2 Two correct ionic half equations scores all 3 marks 3 Diagrams showing electron transfer and charges on the ions scores all 3 marks Fluorine | F2 - PubChem Fluorine is a pale yellow gas with a pungent odor. It is commonly shipped as a cryogenic liquid. It is toxic by inhalation and skin absorption. Contact with skin in lower than lethal concentrations causes chemical burns. It reacts with water to form hydrofluoric acid and oxygen. It is corrosive to most common materials.
Draw the electron dot structure of rmFrm2 fluorine class ... Complete step by step answer: We know that Lewis structures are drawn for molecules containing either covalent bonds or coordinate bonds. These structures are drawn by placing the atoms in an order that less electronegative atom or the atom with the highest valency is kept in the center and more electronegative atoms surround it.
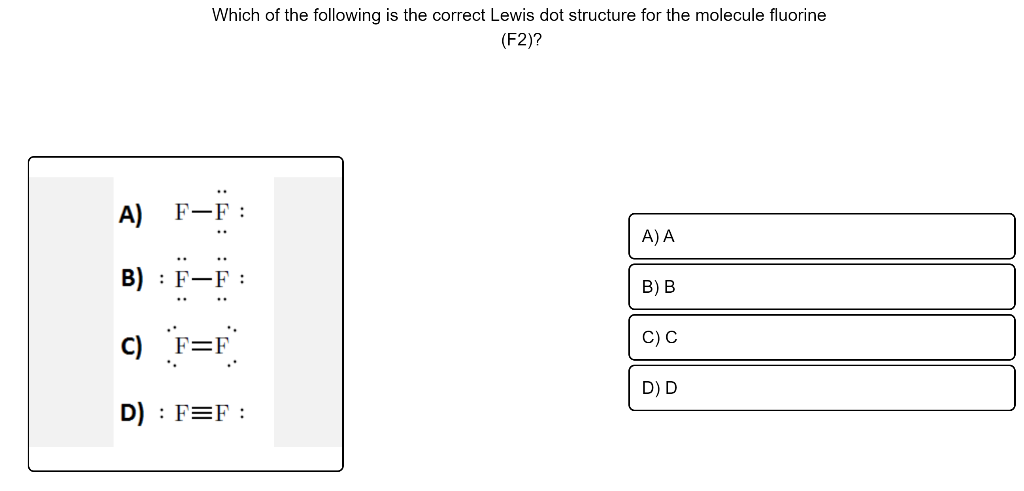
Which is the correct Lewis structure for fluorine, which ... Lewis-dot structure : It is defined as the representation which shows the valence electrons present in an element. The electrons are represented as dots in the structures. The given element is, fluorine which is a group 7A element. The atomic number of fluorine is 17 and fluorine has '7' Get more Answers for FREE
Lewis Structure of PF2Cl3 (With 5 Simple Steps to Draw!) Infact, I've also given the step-by-step images for drawing the lewis dot structure of PF2Cl3 molecule. So, if you are ready to go with these 5 simple steps, then let's dive right into it! Lewis structure of PF2Cl3 contains a single bond between the Phosphorus-Fluorine atoms and Phosphorus-Chlorine atoms. The Phosphorus atom (P) is at the ...
%Fluorine Lewis Dot Structure:Drawing,Several Compounds ... To draw the lewis dot structure of Fluorine gas first have to count all outermost shell electrons in the gas molecule. This number will be 14 as they individually have seven valence electrons. They have to satisfy the Octet rule that each of them must have 8 electrons in valence shell for molecule formation.
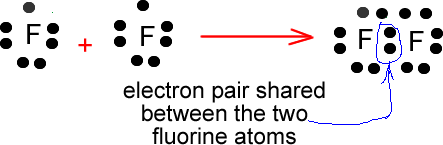
Fluorine (F2) Molecule Lewis Structure There is only one element in fluorine molecule. Fluorine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of fluorine atoms. valence electrons given by fluorine atoms = 7 * 2 = 14 Total valence electrons = 14 Total valence electrons pairs
What is the Lewis electron-dot diagram for a fluoride ion ... Fluorine is in Group 17 of the Periodic Table..... And thus the neutral atom has 7 valence electrons. Of course the elemental form is bimolecular. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?). Which do you think would be bigger; fluorine atom or fluoride ion?
Drawing dot- and- cross diagrams of Covalent Molecules - O ... The dot-and- cross diagram of bromine will look like that of fluorine and chlorine. Only that bromine should be the biggest, followed by chlorine and then chlorine. Dot- and- cross diagram of covalent molecule (oxygen) O 2. Oxygen is in Group VI of the periodic table. This means that an atom of oxygen has 6 valence electrons.
Lewis Structure of GeF4 (With 6 Simple Steps to Draw!) Lewis structure of GeF4 contains four single bonds between the Germanium (Ge) atom and each Fluorine (F) atom. The Germanium atom (Ge) is at the center and it is surrounded by 4 Fluorine atoms (F). The Germanium atom does not have a lone pair while all four fluorine atoms have three lone pairs each.
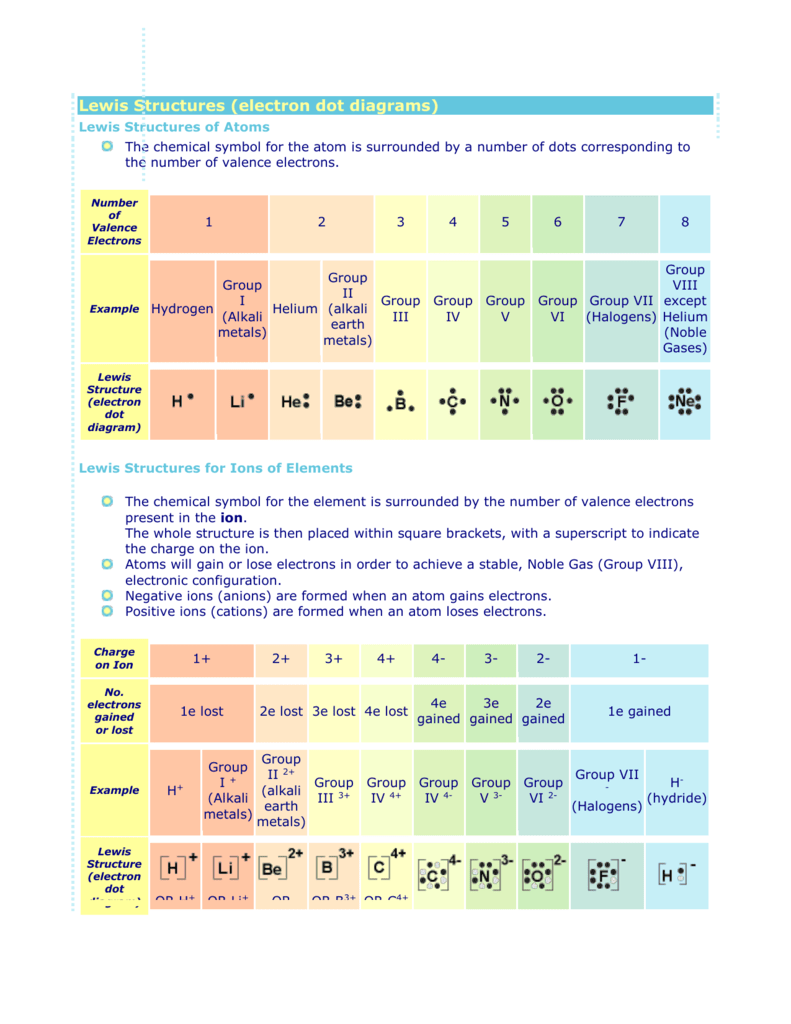
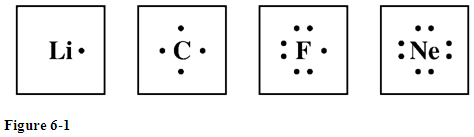
Atomic Protons Neutrons Electrons Lewis Dot Mass Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li
Lewis Structure of HOFO (With 6 Simple Steps to Draw!) Lewis structure of HOFO contains one double bond between the Fluorine atom (F) & one Oxygen atom (O) and the rest other atoms are single bonded with each other. The fluorine atom is at the center and it is surrounded by 1 oxygen atom and one O-H group. Let's draw and understand this lewis dot structure step by step.
Lewis Electron Dot Diagram For Fluoride Ion The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Lithium fluoride, LiF 1. Lithium atom loses one electron to form the cation Li + 2. Fluorine atom gains one electron to form the anion F Lithium fluoride compound can be represented as Li + OR 1.
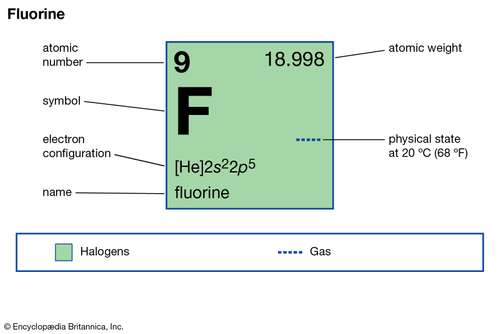
Diagram - Fluorine Fluorine's Diagram. ... Mass Number: 18.9984032 Electron configuration: 1s2,2s2,2p5 or [HE] 2s2 2p5 Valence Electrons: 7 Orbital Notation: Electron Dot Notation: Powered by Create your own unique website with customizable templates. Get Started ...
How to Draw the Lewis Dot Structure for F2 : Diatomic Fluorine A step-by-step explanation of how to draw the F2 Lewis Dot Structure (Diatomic Fluorine).Note that Diatomic Fluorine is often called Molecular Fluorine or ju...
Fluorine Lewis Structure - lewis dot diagram for fluorine ... Fluorine Lewis Structure - 15 images - nf3 lewis structure molecular geometry hybridization, diagram electron dot diagram of fluorine full version hd, structure type sodium fluoride toothpaste, no lewis dot structure youtube,
Sodium Bohr Model - How to draw Bohr diagram for Sodium(Na) atom Electron dot diagram of a Sodium atom. The electron dot diagram also called lewis’s structure which represents the valence electrons of atoms. As, from the Bohr diagram of Sodium, we got to know, it has only 1 valence electron. So, just represent the 1 valence electrons around the Sodium atom as a dot.
[Solved] Match each element to its electron dot diagram ... Match each element to its electron dot diagram. The symbol X represents the element. Refer to the periodic table if needed. fluorine sodium phosphorus magnesium One of the X's didn't work, but it had 2 dots on top, 2 on the right, 2 on the bottom, and 1 on the left.
Electron Dot Diagram For Fluorine - schematron.org Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?).Study the electron dot diagrams for lithium, carbon, fluorine, and neon in figure Choose the statement the correctly identifies the most stable of the elements. a. lithium b. carbon c. fluorine d. neon.
SF6 Lewis Structure, Molecular Geometry ... - Techiescientist 2 days ago · In the outermost shell, we have 6 electrons that are also known as valence electrons in the case of Fluorine. Lewis Dot Structure of SF6. The central atom here is Sulfur as it is less electronegative than Fluorine. This is because the outer shell of Fluorine has 5 electrons and it needs one more electron to reach stability, which is easier to ...
Build an Atom - Atoms | Atomic Structure | Isotope ... - PhET Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
Lewis Dot Structure for Fluorine Atom (F) - YouTube A step-by-step explanation of how to draw the Lewis dot structure for F (Fluorine). I show you where Fluorine is on the periodic table and how to determine ...








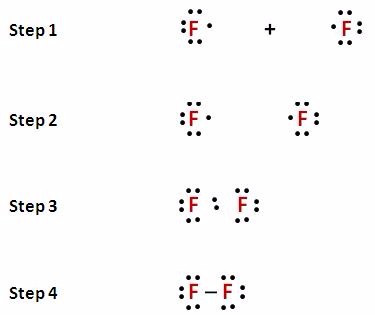







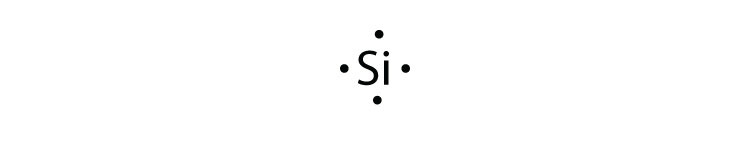





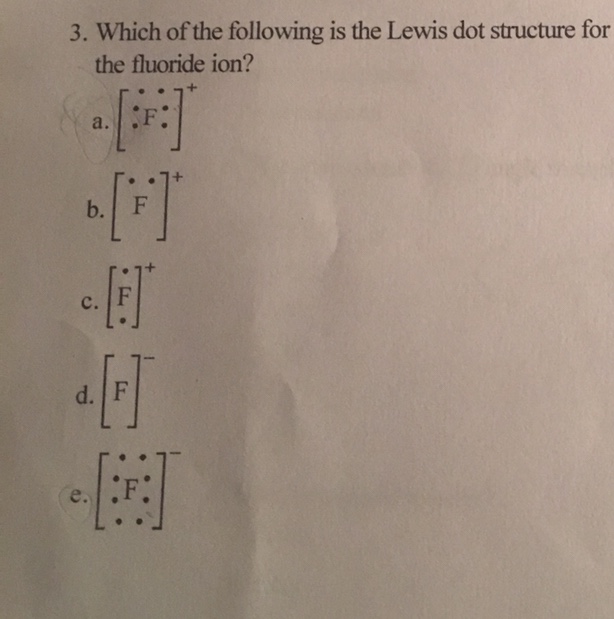

0 Response to "40 fluorine electron dot diagram"
Post a Comment